The title and original concept for this paper is credited to my
colleague Dr Anthony Rees.
Commercial Indication
Homoeopathic Products:
State Sanctioned & Taxpayer Sponsored Health Fraud!
PSEUDO-SCIENTIFIC
HOMOEOPATHIC PRODUCT MANUFACTURING COMPANIES ARE HIDING BEHIND FALSE
ADVERTISING AND PREJUDICING THE ESTABLISHED SCIENTIFIC HEALTH AND
THERAPEUTIC POTENTIAL OF NUTRITIONAL AND HERBAL PRODUCTS, TO FRAUDULENTLY
PEDDLE THEIR PLACEBO PRODUCTS AS MEDICINES, WITH SERIOUS UNSUBSTANTIATED
INDICATIONS AND EFFICACY CLAIMS, WITH STATE SANCTION, AND AT TAXPAYER'S
EXPENSE.
The proposed “listings system”
(Expedited registration procedure- ERP), initiated, dominated and
driven principally by the “big three” natural pharmaceutical
homoeopathic pseudo-medicine companies, is a natural health suppressive
and monopolistic GMP-based regulatory initiative, inappropriately
favouring financial might and impractical quality rather than safety
/ efficacy criteria.
THE POLITICS
The Dukes Review Report, whose two external
experts, not coincidentally hailed from the only countries currently
enforcing the listing system, strategically endorsed what was initiated
by disgraced former MCC chairman Folb, in line with a developing
WHO pharmaceuticalisation / harmonisation policy.
This will prejudice nutrition and herbalism via a self-favouringhomoeopathy-driven
compromise whereby the least scientific modality
benefits disproportionately by the regressive policy position that
"the criteria of demonstrated
efficacy will be replaced by ‘evidence’ that the medicine
is used within a particular philosophy or tradition for particular
purposes", thereby missing the central objectives
of medicines regulation. Ironically, o-t-c homoeopathic indication,
and especially the combination products, do not strictly qualify
as homoeopathy. A concomitant compromise is that "the
criteria for reliable information will be modified so that claims
can be accepted which do not transcend certain specified limits",
and specifically "no reference should be made to resistant
conditions, major infectious diseases, asthma, cancer and epilepsy".
Whilst it is obvious (based
on the scientific evaluations presented) that these latter
limitations are entirely appropriate for over-the-counter combination
homoeopathic products, they are inappropriate, indeed devastatingly
prejudicial to both nutritional and herbal products. Whereas
considerable real scientific validation exists for nutritional and
herbal substances, and this expands chrono-exponentially, the opposite
pertains to homoeopathic medicines, which are
still struggling with hypothetical therapeutic rationale, and have
yet to convincingly establish significant therapeutic efficacy for
a single clinical condition
During the apartheid era, homeopathic remedies enjoyed
a unique status in the health market-place, being largely unregulated
until the mid-80's, and for the next decade illegally enjoying pseudo-registration
status whereby product application numbers were allocated,
but registrations never processed further, since no efficacy data
existed, but yet these applications were never cancelled, and these
products fraudulently remain on the market with totally unsubstantiated
serious indication claims, putting consumers at considerable risk.
Subsequent to the democratic elections, the post-sanctions era heralded
a flood of nutritional and herbal products onto the local market
in competition with the local homoeopathic companies, who reluctant
to relinquish their apartheid-gained monopolies,
increased familiarities with the now disgraced former MCC hierarchy
and via the HPA executive, despite financial vested interests, negotiated
the terms of reference for the listing system to preferentially
suit their own local circumstances and pharmaceutical company status.
In South Africa today, only homoeopathy enjoys
the benefits of taxpayer's money by means of grants to
it's training faculties, in spite of it being the least
scientific of all the complementary modalities. In the
mid-70’s, the Allied Professions Board closed all courses
teaching self-reliant homeopathy, naturopathy, and herbalism. A
decade later two Technikons opened faculties exclusively teaching
non-classical pseudo-homeopathy, with syllabi essentially teaching
biomedical homoeopathy, a soulless hybrid in conflict with the Hahnemannian
tradition. Recent graduates, no longer making their own remedies,
now resort to purchasing commercial stock from the big companies.
After 25 years, herbalism nearly became extinct, since with the
exception of personal favours and admissions of previously disadvantaged
unqualified students for political expedience, not even internationally
qualified herbalists were granted registration by the new Interim
Allied Professions Council, still openly exercising ideological
bias in favour of homoeopathy and against herbalism.
THE REALITY
Homeopathy dates back to the late 1700s
when Dr Samuel Hahnemann began formulating its basic principles,
based on provings which have been in use for about 175 years without
substantial revision. Even recent provings are of highly questionable
quality, not to mention value. The doctrine is not and can never
be a theory of physiology or of the effects of drugs on the organism
and pathological processes. Homoeopathy's elaborate symptomatic
descriptions require an extreme degree of individualised case-taking.
The homoeopath has little leeway in the remedy selection and must
at all times be guided by the (totality of) the symptoms (1).
Whatever is not compatible with
Hahnemann's three rules is excluded from homoeopathy, which
advocates the single remedy since the provings are never
of mixtures (1). Indication products cannot qualify
as homoeopathy. Homoeopathic success is attributable primarily to
spontaneous remission, the healing power of the compassionate and
reassuring consultation (1-3 hours), plus the power
of placebo (belief), which are collectively estimated to
contribute some 70-100% of observed benefits in controlled trials,
and all of which are negated with the use of such products. This
author believes that the practitioner's desire to relieve suffering
has a synergistic effect, according to the maxim: "energy follows
thought". The author is utterly convinced, on the basis of
the latest scientific research, that the homoeopathic remedy
itself has no intrinsic effect. This conviction is confirmed by
negative results in the most rigorous trials.
The author's position on
the mere ritualistic value of homoeopathic remedies are borne out
by the results of placebo statistics and
meta-analysis of randomised placebo-controlled trials of homoeopathy
which show that placebo
(nothing) works better than the remedy. The
most recent and comprehensive 1997 meta-analysis of 89 strict-criteria
randomised placebo control trials by a German university Centre
for Complementary Medicine Research concluded that there was
“insufficient
evidence that homoeopathy is clearly efficacious for any single
clinical condition” (2), the complex homoeopathic
remedy epitomised.
The laws of chemistry state that there is
a limit to the dilution that can be made without losing the original
substance altogether, (Avogadro's number), which corresponds to
homeopathic potencies of 12C or 24D(X). A 30X dilution means
that the original substance has been diluted 1,000,000,000,000,000,000,000,000,000,000
times. To get even one molecule of the substance in the most common
30X pills, would necessitate taking two billion of them, about a
thousand tons of lactose tablets (or one hundred tons of drops).
Even under the most scrupulously clean conditions, airborne
dust in the manufacturing facility carry thousands
of different extraneous molecules of terrestrial and even
extraterrestrial origin. Similarly, the "inert" diluents
used in the process have their own vast variety of micro-contaminants.
How does the emerging preparation differentiate as to which of the
molecules present are intended to be potentised?
References
(1) Coulter H, Homoeopathic Science and Modern
Medicine. Amer. Inst. of Homoeopathy / N. Atlantic Books, 1980;
(2) Linde K, et al, Are the clinical effects
of homoeopathy placebo effects? Lancet 1997 Sep 20; 350(9081).
After evaluating all scientific reviews
of homoeopathic trials to date, even though the remedy 'appears'
in many cases to perform beyond mere placebo, one has to conclude
that the spontaneous remission / placebo complex, commonly and hereafter
simply termed placebo (nothing), in the final analysis, is at work
rather than the actual remedy itself. This is based logically on
the scientifically indisputable (measurable and reproducible) existence
of a reliably powerful placebo effect. (1), (2),(3),(4),(5)&(6),
whereas conservative elimination of the confounding trial factors
comprising considerable methodological flaws and significant publication
bias (7-19), reduces any supposed favourable evidence to mere false-positives,
also confirmed by subsequent rigorous trials.
I shall substantiate my taking care to choose
only publications and authors known to be objective in the evaluation
of complementary medicine. Data searches encompassed all published
reports of controlled clinical trials, including journals, books
and conference proceedings, as well as reviews and meta-analysis,
covering all countries and all homoeopathic types and potencies.
Overall, there were considerable positive results, especially in
earlier studies, but progressively controlling for confounding factors
by correctly making trials more rigorous has resulted in the scientific
conclusion by homoeopathic advocate scientists, that there is insufficient
evidence for the efficacy of homoeopathic medicines for even a single
clinical condition (13) (which is the application of complex remedies
bearing disease indications / claims). Observe the steady evaporation
of presumed evidence.
Investigation started with the earliest
comprehensive 1984 review by Scofield,
“Experimental research in homoeopathy - a critical review”
(7), which concluded that, "It is obvious that despite
much experimental and clinical work, there is only little
evidence to suggest that homoeopathy is effective. This
is because of bad design, execution, reporting, analysis and particularly
failure to repeat promising experimental work and not necessarily
because of the inefficiency of the system which has yet to be properly
tested on a large enough scale. There is sufficient evidence to
warrant the execution of well-designed, carefully controlled experiments.
Homoeopathy has most certainly not been disproved.” Before
advocates celebrate this tit-bit, they are reminded that there is
more to come and that it is the absence of proof, rather than the
absence of disproof that matters.
As Scofield concluded: “It is
hardly surprising in view of the quality of much of the experimental
work as well as its philosophical framework, that this system of
medicine is not accepted by the medical and scientific community
at large.” A 1990 “Review of randomised
trials of homoeopathy” by Hill and Doyon
(8), covering published European studies and a wide range
of pathologies, did “not provide acceptable evidence
that homoeopathic treatments are effective.”
Out of 40 randomised trials, all but three had major design flaws
and only one of these had reported a positive result. (8) Published
in a French journal, this review received little attention outside
France, especially since the conclusion was that “proof
for efficacy is inadequate” (9)
A contemporary English review by Kleijnen
et al (10) disagreed, including two trials considered to
be non-randomised and seven negative by Hill and Doyon
as randomised and positive (9), and concluding that “on the
basis of the existing evidence, they would be ready to accept that
homoeopathy can be efficacious if only the mechanism of action would
be more plausible.” (10) The Kleijnen review
“became the paper of reference, even though it was criticised
for two shortcomings, in particular: 1) In the quality assessment,
a crucial issue of methodological quality - handling of drop-outs/withdrawals
– was not included; 2) The method of categorising results
into ‘positive’ and ‘negative’ is open to
bias and leading statisticians do not recommend this.”
(9) Kleijnen, an authority on alternative medicine, as principal
author,himself admitted several shortcomings. (10)
Kleijnen
et al in their
1991 BMJ review (10)
“Clinical trials in homoeopathy”
commented as follows: "The results of all
studies may be seriously biased because of several methodological
shortcomings. In 42 of 107
trials, there was insufficient data to check the often over-optimistic
interpretation of the outcome(s). Overall, the quality was disappointing.
Sometimes only some of several interventions, measurements of outcome,
or data presentations met the criteria. Only
23 scored greater, and 84 less than 55 for the maximum of 100 for
quality. With limited participants (often not mentioned)
(less than half had over 25 patients per group), one cannot be confident
that randomisation will equally divide known and unknown confounders".
(10)
"Publication
bias is an important problem. Only 17 described the method of randomisation.
Whilst 75 were double blind trials, placebo
was 'described' as indistinguishable in only 31. Patients
have many ways to break the code, which might explain any differences
in favour of homoeopathy. Double blinding was not checked
in any trial of homoeopathy. The
process of producing preparations and their composition, especially
herbs, differs greatly among manufacturers and hence preparations
may still have pharmacological effects since it
is sometimes difficult to demarcate phytotherapy (Prob.>1C/2D-2C/4D)(ST))
from modern homoeopathy".
(10)
"A trial of very high quality by
the Groupe de Recherches et d' Essais Cliniques en Home'opathie
initiated by the French Ministry to retest (apparently
positive) results in a new rigorous trial, found no positive
evidence for homoeopathy” (11). “Will more
such trials refute the existing 'evidence' ?", asked Prof.
Kleijnen.(10) Boissel et al of the 1996 Homoeopathic Medicine
Research Group, in report titled “Critical
literature review on the effectiveness of homoeopathy: overview
of data from homoeopathic medicine trials” reflected
this dismal state of affairs when they stated that “after
examining 184 reports of controlled trials, they considered only
17 to be worth considering” and concluded: “the
number of participants was too small to draw any conclusions about
the effectiveness of homoeopathic remedies for any specific condition.”(12)
Dr. Klaus Linde,
principal author of the comprehensive 1997 Lancet
meta-analysis, “Are the clinical effects of homoeopathy placebo
effects? A meta-analysis of placebo controlled trials”,
(13) (Centre for Complementary Medicine Research, Munich, FRG),
authored a rave BMJ review of research on St. John's Wort for depression.
The final author (13) was Dr. Wayne Jonas
(Director, Office of Alternative Medicine, National Institutes of
Health, USA). Funding included the pro-homoeopathic Carl and Veronia
Carstens Foundation, Essen, FRG. (13) Acknowledged were the contributions
of the documentational centres of Boiron, Dolisos and Heel. To placate
sponsors, the results were interpreted as "not compatible with
the hypothesis that the clinical effects of homoeopathy are 'completely'
due to placebo", with an honest bottom line: "We
found insufficient evidence ( in 185 trials) that homoeopathy is
clearly efficacious for any single clinical condition".
(13)
Elation at the placatory result was further
deflated by an under-reported analysis in Prescrire
International announcing that: “A thorough
examination of this meta-analysis reveals design errors
that make the results untrustworthy. There is
nothing to suggest that homoeopathic drugs are any more effective
than placebo”. (14) What Linde et al found and why.
"The combined odds ratio for the 89 studies entered into
the main meta-analysis was 2.45 in favor of homoeopathy, (reduced
to) 1.66 for the 26 best-quality studies. The ratios were computed
such that a result greater than 1 indicated greater effectiveness
of homoeopathy. A combination of publication
bias and poor-quality trials and/or other factors unaccounted for
might have led to erroneous results. The evidence
in our overall analysis would be more compelling if there were independently
replicated, large-scale rigorous trials of defined homoeopathic
approaches in at least a few specific disorders". (13)
To put this into perspective, a review in
the journal Bandolier:
Evidence-based health care, which favourably reviewed Kleijnen'
s ginkgo and Linde's St John's wort papers, described the results
thus: "This will be interpreted by some as signifying that
homoeopathy works, but in
60% of trials, homoeopathy could not be shown to have any benefit
over placebo. If this were a new treatment, we would look
at it with a very cold and fishy eye. A
skeptic might say, if this is the best they can do, why bother ?".
(15) Bandolier provided a comparative quantative analysis of the
clinical categories: Overall,
placebo alone beat placebo plus homoeopathy in 6 out of 10 (58%)
of the trials. Where homoeopathy minimally added
to placebo (allergy, neurology, rheumatology and miscellaneous),
the ratio was only 4 to 3, but
where placebo beat homoeopathy, the ratios significantly
favoured placebo: dermatology 6/3, gastroenterology 6/3, muscoskeletal
4/2, chest infection, asthma, ENT 11/4, and surgery and anaesthesia,
8/4, all in favour of placebo. (15) (100% superiority)
"Quality
of evidence is a major problem, the mean quality
score being 52%. About 2/3 were poor, 1/10 good. Many
trials by advocates with high enthusiasm risks incomplete and selective
reporting. Major shortcomings
were evident on the clinical level. Inadequate peer-review allows
other undetected 'fatal flaws'. Overall quality-assessments
can mix and obscure confounding, eg. unequal
distribution of prognostic factors might explain positive
results; knowledge and expectations
about receiving 'active' treatment can bias judgements during reporting
or measurement of outcomes; dropouts, withdrawals, or inadequate
follow-up can result in unequal distribution of results between
groups not due to treatment effect; and multiple outcome-measures
or post-hoc selection of outcomes can lead to reporting false-positives.
No trials met our criteria
for reproducibility". Of only three qualifying
industry inclusions, the combined quality scores were 48.5, 31.5
and 24 out of 100. (13)
"Patients, physicians, and purchasers
need valid and reliable information (unencumbered by opinion) on
which to make decisions. Whilst randomised placebo-controlled trials
hold an important place in such decisions, it
is likely that higher quality trials in homoeopathy will show less
significant results. We
found little evidence of effectiveness for a single homoeopathic
approach on any single clinical condition. In
the end homoeopathy may be found to have no value".
(13) In subsequent correspondence, Linde
and Jonas respond
to three letters to the editor enthusing the data: "We
do not share the enthusiasm. The evidence is not overwhelming".
(16) Responding to prior data of this nature, a London health authority
recently stopped paying for homoeopathic purchases after a decision
to support only evidence based medicine led to a review of recent
research, including that by the Royal Homoeopathic Hospital,
which produced no evidence of clinical benefit.
(17)
In the Lancet, Prof. M Langman, (Univ
Birmingham) commented: "Only 34 trials showed adequate evidence
of concealment of treatment allocation and 28 sufficient handling
of drop-outs". (13) In a subsequent Lancet, Dr. A Koch,
(Univ Heidelberg) wrote: "Where
there is no concealment, two placebos might well differ with respect
to efficacy if there is one in which one can belief more".
(16) In the BMJ, Dr. M Francis-Kahn (Me'decin de l'Hospital Bichat,
Paris) wrote: "One
can challenge results obtained with dilutions retaining some active
molecules and high dilutions in which no active
molecule is present and results
presented by a homoeopathic drug company. A negative report by Kleijnen
is in Linde's meta-analysis positive (yet) Andrade's overall conclusion
is negative. The
report by Fisher (Research Director, Royal London Homoeopathic Hospital)
was so poor that a critical study was published in the Lancet showing
the inappropriate use of statistics. With
respect to the negative best controlled study by French health authorities
to confirm or contradict two previous quite poor reports, it is
unfair to write that the pooled effect was in favour."
(17)
“Publication bias is
a significant problem and occurs when the chance that a trial is
reported depends to some extent on the outcome of the trial. We
cannot completely rule out bias as an explanation for positive results.
Funnel plot of log odds ratios versus their standard errors has
been widely used to detect potential publication bias. The asymmetry
indicates missing negative trials. The general non-parametric selection
model applied to the 89 studies confirmed that there was statistically
significant publication bias and suggested this was due primarily
to under-reporting of studies with statistically insignificant effects
and with negative effects”. (13) In the Lancet,
Prof. J Vandenbroucke (Univ. Leiden) commented: “A
randomised trial of ‘solvent only’ versus ‘infinite
dilutions’ is a game of chance between two placebos.
The authors used a funnel plot to look at the results. If
there is publication bias, there should be a gap on the negative
side of the plot. Linde et al find a bunch of outliers among the
positives”. (13) See next paragraph / page for
funnel plot.
In this regard, Vandenbrouke in the BMJ
petitioned for experts’ views, pointing out that “Egger
et al’s funnel plot test predicts that there might be a problem
because the funnel
plot is asymmetrical and that the cause of the asymmetry can be
anything from publication bias, willingness to please during data
collection, data massage in the analysis, downright fraud or a mix
of these”. Matthias
Egger (Univ Berne, Switzerland) responded: “Results
of meta-analysis will depend on how many small or large studies
are included (more positive results in smaller trials). Vandenbroucke
could have benefited from a formal analysis of funnel plot asymmetry
when he discussed a recent meta-analysis on homoeopathy (13), since
the significant funnel plot asymmetry lent support to his assertion
that bias had produced a body of false positive evidence”.
(18) The article’s accompanying figure of the asymmetrical
funnel plot signifying bias, is provided below.
BMJ
No 7129 Volume 316, Letters, Saturday 7 February 1998
Bias in meta-analysis detected by a simple, graphical test.
Bias in meta-analysis is often reflected
in asymmetrical funnel plots. Vandenbroucke could have benefited
from a formal analysis of funnel plot asymmetry when he discussed
a recent meta-analysis of homoeopathy. (1) Significant funnel plot
asymmetry (P0.001) (would have)
lent support to his assertion that bias had produced a body of false
positive evidence (fig). (2)
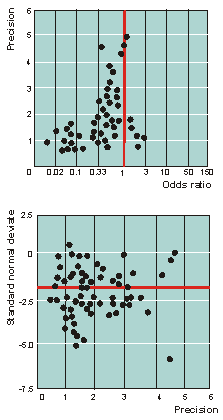
Fig 2.
Asymmetrical funnel
plot of clinical trials of homoeopathy (upper panel) indicating
presence of bias. The linear regression of the standard
normal deviate against precision (defined as the inverse of the
standard error) shows a significant (P0.001) deviation of the intercept
from zero (arrow). In the absence of bias, trials would scatter
about a line running through the origin at standard normal deviate
zero.
Matthias Egger, George Davey
Smith, University of Bristol.
Christoph Minder Head, University
of Berne.
Funnel Plot References:
(1) Linde K, Clausius N, Ramirez G, Melchart
D, Eitel F, Hedges L V, et al. Are the clinical effects of homoeopathy
placebo effects? A meta-analysis of placebo-controlled trials. Lancet
1997; 350:834-43.
(2) Vandenbroucke J P. Homeopathy trials:
going nowhere. Lancet 1997; 350:824.
Prof.
E Ernst, holds the
world’s first permanent Chair in Complementary Medicine, (Dept.
Compl. Med. Univ. Exeter, UK). Prof. Ernst has published positively
in medical journals on eg. garlic, St John’s wort and yohimbe;
extensively on placebo and on safety and efficacy of complementary
medicines, and has authored textbooks on complementary medicine,
garlic and homoeopathy. (19) In the Lancet he responded as follows:
“We compiled data from trials
of homoeopathy published
after Linde and collegues’ searches were completed.
Linde mentions two, both
of which were negative. We found four further reports and the only
common factor is that none
of them show any superiority of homoeopathy over placebo.
Furthermore, a recent systematic
review of seven controlled trials of homoeopathy for a condition
judged non-clinical by Linde, included three
randomised controlled trials, all of which reported negative results
for homoeopathy. The
picture painted by Linde may well be slightly more positive for
homoeopathy than recent published evidence implies”.
(16)
The most commonly quoted allegedly positive
homoeopathic trials are those of Reilly D (Lancet
1994; Dec) and Jacobs J (Pediatrics,
1994; May). Both have been methodologically criticised, yet are
still widely quoted. Reilly’s paper was criticised by
Plasek and Zvarova. The treatment was not homoeopathic,
but isopathic and the reliability of the trials analysed called
into question. (20) Jacob’s study was criticised by Sampson
and London: 1) it used an unreliable and unproved diagnostic
and therapeutic scheme, 2) there was no safeguard against adulteration,
3) treatment selection was arbitrary, 4) the data were oddly grouped
and contained errors and inconsistencies, 5) the results had questionable
clinical significance, and 6) there was no public health significance
because the only remedy needed for childhood diarrhoea is adequate
fluid intake/ rehydration. (21) Just because an article appears
in a scientific journal does not mean that it should be accepted
and incorporated into therapeutic regimens. It is only published
initially for critique and review for possible further research.
Kleijnen, Boissel, Linde, and Ernst are
all researchers who have in common an interest in complementary
medicine taking its rightful place in health care, which is only
possible if evidence-based. They are recognised authorities in their
respective fields and are key members of the Cochrane Complementary
Medicine Field. Cochrane Centres world-wide are evaluating
both paradigms according to the available evidence. Dr. Ian Chalmers,
Director of the UK Centre, a vociferous proponent of systematic
reviews, illustrated their objectivity when he told a conference
on integrated medicine in London recently that “Critics of
complementary medicine often seem to operate a double standard”
and that “the aim should not be to indulge in data-free arguments,
but to assess the effectiveness and safety of any healthcare intervention,
be it orthodox or complementary”. (22)
References:
(1) The Placebo Response: Biology and Belief,
Conference, Univ Westminster, Nov 1996;
(2) OAM Placebo and Nocebo Conference, Office
of Alternative Medicine, NIH, Dec 1996;
(3) The Placebo Effect: An Interdisciplinary
Exploration, A Harrington, Ed, Harvard Univ Press 1997;
(4) Brown W. The Placebo Effect, Scientific
American, Jan 1997,
(5) Shapiro A & E, The Powerful Placebo,
John Hopkins Univ Press 1998;
(6) Vincent C, Furnham A, Complementary Medicine:
A Research Perspective, J Wiley & Sons 1998;
(7) Scofield A, British Homeopathic Journal
1984; 73(4);
(8) Hill C, Doyon F, Rev Epidemiol Sante Publique
1990; 38(2);
(9) Linde K, et al. Overviews … of controlled
clinical trials of homoeopathy. In Ernst E, Hahn E, 1998;
(10) Kleijnen J et al, British Medical Journal
1991, Feb 9; 302(6772);
(11) GRECHO. Presse Med, 1989; 18,
(12) Boissel, J. et al, HMRG. Report to the
Europ Comm, Brussels 1996;
(13) Linde K, et al, Lancet 1997; Sep 20;
350(9081);
(14) Prescrire International 1998 Jun; 7(35);
(15) Bandolier No 45, Nov 1997;
(16) Lancet, 1998; Jan 31; 351(9099);
(17) British Medical Journal, 1997; May 31;
314 (1569);
(18) British Medical Journal, 1998; Feb. 7;
316(469);
(19) Ernst E, Hahn E, Homoeopathy, a critical
appraisal, Butterworth Heinemann, 1998;
(20) Plasek J, Zvarova J, Cas Lek Cesk, 1996
Sep 18. 135(18);
(21) Sampson W, London W, 1995 Nov; 96(5 Pt
1);
(22) British Medical Journal, 1998; June 6;
316(1694).
Previous articles in this series proved
quite conclusively that homoeopathic remedies are worthless beyond
their singular ritualistic value. The local homoeopathic fraternity
were invited to present any evidence to the contrary, but either
declined or subsequently withdrew their efforts as the strength
of this thesis became evident. Similarly, the threats of legal action
evaporated as the truth of this position set in.
It was originally the intention to expose
only the monopolistic and fraudulent acts being perpetrated by the
big homoeopathic companies from behind a sickening charade of public
beneficence, but subsequent denial by homoeopaths themselves and
refusal to consider evidence led to the publication of proof of
their delusion. This led to even deeper denial as their peculiar
cultic faith, and or ego's (besides considerations of financial
concern) stood in the way of honest reappraisal and acceptance of
the facts of solid wholistic science, presented in the main by actual
proponents of homoeopathy and complementary medicine.
John Davidson, a highly respected modern
esoteric author noted: "It is one of the most important, yet
most neglected discoveries of medicine that 'nothing' will actually
cure, regularly and frequently". (1) In a British homoeopathic
journal he wrote that "In homoeopathy, the issue may be even
more complex: Homoeopathy it is often claimed, works through enhancing
the self-healing processes; this could mean that homoeopathy simply
maximises the placebo response". (2) Davidson has further written
that "Even pathological and physiological symptoms can disappear
when the individual's mind is convinced. If the mind is convinced
ill-health will continue, then all the drug-molecules in the world
will not help". (1)
Prof. Dr. W. Gaus and Dr Hogel (Univ. Ulm),
developed a homoeopathic trial design which takes into account the
individual selection of classical homoeopathic medicines. In a double-blind
trial in patients with chronic headache, after two months of such
treatment, patients suffered from headache on fewer days, duration
of headache was less severe, and intake of analgesics had been reduced.
Not bad for homoeopathy, generally not very successful with headache.
However, therapy was equally successful in the placebo group. (3)
Is it really so wrong to expose how much of healing, (incl. orthodox),
is placebo?
A recent example of blind enthusiasm is
a feature in the local publication, 'Health Independent' (Sept 98),
which ran a propaganda piece titled "Homoeopathy gaining acceptance
throughout the world: AMA journal publishes positive study of homoeopathic
medication for vertigo". The text implied that finally being
featured in medical journals, attributed scientific credibility
to homoeopathy, whereas anyone remotely honest would have to reach
the opposite conclusion. The cited Lancet and BMJ (isopathy) and
Pediatrics studies have been subsequently refuted due to flawed
methodologies, and the Lancet meta-analysis failed homoeopathy on
the same criteria, plus established no efficacy for any single application.
Significantly the obscure AMA Archives of
Otolaryngology paper was a comparison of Vertigoheel
with betahistidine as an equivalence control, rather
than with placebo. Furthermore the study was unorthodox in that
it was conducted by the manufacturers: Heel Inc,
and this story lifted off their commercial web-site. Most telling
however, is that betahistine is described as "standard conventional
therapy" and Vertigoheel as being "as effective",
yet the spokesperson, also the principal author, goes on to reveal
the illusion of efficacy by stating that "because
of the lack of effective conventional treatments, Vertigoheel fills
a serious void", but thereby logically admitting that the homoeopathic
treatment was as effective as a non-effective conventional treatment.
Enter spontaneous remission and placebo and hey presto: efficacy!
Vertigoheel, a combination clinical so-called
homoeopathic medication, interestingly does not strictly qualify
as such, since in the manufacturer's own words "unlike classical
homoeopathic drugs, the active ingredients in Vertigoheel are not
ultra-highly diluted and the pharmacological and clinical profiles
can be defined within the conventional medical paradigm, a bridge
between homoeopathy and conventional pharmacology". Furthermore,
I note that the most concentrated active (D3)(Conium) is a potent
toxin and is within a range where it admittedly functions pharmacologically.
The 70%
improvement attributed to both 'active' treatments is however also
well within the same range of that expected from a good placebo.
Over and above the refuted evidence from
homoeopathic clinical trials, really weak arguments include 'evidence'
from case studies, materia medica 'provings' (observations), and
healing with animals, which simply do not constitute an iota of
scientific evidence, since the circumstances and numbers are not
only inadequate, they are a joke, and spontaneous remission (we
are all self-healing organisms) and placebo effects easily cover
the observations. Animals also respond to care and concern and professor
Ernst, Chair of Complementary Medicine at the University of Exeter
has described the animal argument as "weak". (4)
Science has not embraced homoeopathy, and
for good reason. New Scientist Magazine commented on the recent
Linde et al homeopathic meta-analysis as follows: "A few teams
failing to publish a negative trial; a few claiming they tested
the remedy blind when in fact they were aware which patients were
getting the remedy and which the placebo, and hey presto, homeopathy
nudges ahead in the pooled analysis" (5). In a recent Scientific
American article, Walter Brown (psychiatrist) of Brown University
School of Medicine commented that: "Although alternative medicine
healers and their patients believe fervently in their effectiveness,
many of these popular remedies probably derive their benefit from
the placebo effect". (6)
75 - 90% OF ALL MEDICINE
IS PLACEBO
Most people who think that they do, don't
truly understand what the placebo effect is. Spontaneous remission
and the placebo effect, known as nonspecific effects, are significant
phenomena that have veiled impact. The major logical error in plotting
disease progress is: post hoc, ergo propter hoc
("after and therefore because of"). This common fallacy
credits improvement to a specific treatment merely because the improvement
followed the treatment. Placebo is best understood in terms of the
common factors associated with various types of therapy, such as
expectancy, contact with a therapist, and therapeutic alliance.
Not only medication, but also other features of the physician-patient
encounter may recruit the healing response. Careful analysis may
be far more comforting than immediate diagnosis. (6)
The use of a placebo group is now widely
considered by scientists to be crucial in demonstrating that the
observed improvement is not the result of the incidental aspects
of treatment. The adoption of the randomized, placebo-controlled
trial (provided that statistical significance is not falsely P-valued,
but is rather analysed using Bayesian methodology) ensures an elegant
control, since experimenter or patient bias or a confound of patient
differences with treatment method may be respectively countered
by double-blinding and randomization. Although orthodoxy controls
for placebo, almost no one evaluates them, yet significantly, more
placebos have been administered and confirmed than for any experimental
drug. (ST) Some perceptive scholars believe that the history of
medicine is the history of the placebo response. (7)
The standard textbook 30% for placebo is
unrealistic low. Strauss and Cavanaugh showed placebo response rates
for some psychiatric disorders: major panic disorder 51%; depression
67%; & generalized anxiety disorder 82%. (8) A recent conference
reported that 50-72% of the children in a Ritalin-
Placebo evaluation, were rated as being improved while on placebo
in both the home and school environment regarding the severity of
problems, and the number of problems demonstrated. (9) Verdugo and
Ochoa, noted that after diagnostic intervention, pain/hypoaesthesia
was relieved in 66,6% of patients. (10) In its most general sense,
"placebo" includes spontaneous remission, the patients
belief, the healer's 'energy follows thought' contribution and other
incidental factors. Medicinal efficacy are exclusive effects, if
any.
Kirsh and Sapirstein, Ph.D's at Univ. Connecticut
and Westwood Lodge Hospital, MA, respectively, using meta-analysis
to evaluate the magnitude of the placebo response against 16 antidepressant
medications (including Prozac) in 19 strict criteria
double-blind clinical trials with 2,318 patients, determined that
the inactive placebos produced improvement of 75%
of the effect of the active drug. They concluded that "experiencing
more side-effects, patients in active drug conditions
concluded that they were in the drug group; and this can be expected
to produce an enhanced placebo effect in drug conditions and thus,
the apparent (additional) drug effect may in fact be an active placebo
effect". (11)
Larry Beutler, University of California,
added: "translating the mean placebo response effect size reveals
that 88% of patients who received only placebos
experienced improvement (12% stayed the same or got worse) and only
15% gained benefit by antidepressants over placebo alone.
To some it might appear obvious that the front line treatment
of choice is placebo, not antidepressants". He also
commented: "Collectively, the poor showing of antidepressants
in this and other meta-analytic studies raise an interesting
question about why and how public enthusiasm and faith is maintained
in these treatments, a research question whose importance may even
exceed that of the effects of the drugs themselves".
(12)
Beutler opinioned that "One may wonder
whether the increase in the number of drug patients improved is
worth the cost. These results challenge certain widely held beliefs
about the effectiveness of medication and have direct relevance
for questions about the adequacy of contemporary methodologies to
control for the effects of expectation, hope, and nonspecific treatments".
(12) Kirsh stated that "Although our data do not prove antidepressants
to be ineffective, it does indicate that effectiveness still needs
to be established". (13) The same for homoeopathic medicines,
which to date have not achieved any proven success. Any statistical
significance is negated by Bayesian analysis to
standard arbitrary P-value results.
Dr Andrew Weil M.D. points out that "in
1842 Oliver Wendell Holmes (echoing Voltaire) wrote that the fact
of homeopathic cures should not be admitted as evidence, because
90% of cases commonly seen by a physician would
recover sooner or later, with more or less difficulty, provided
that nothing were done to interfere seriously with the efforts of
nature". Weil adds: "In other words, most sick people
will get better no matter what you do, as long as you do not actively
make them worse, a strong argument, consistent with the experience
of most observers of illness, (and concludes that) we may quibble
over the percentage of cases that will recover anyway, but it is
certainly high, and may well be as high as 90%". (7)
THE ETHICAL SOLUTION
Dr Robert Becker M.D. writes: "The
minimal techniques of energy medicine are quite different from the
placebo effect as depicted and condemned by orthodox medicine. The
body's internal energetic systems may be accessed by the conscious
mind through the use of several techniques that do not involve the
addition of any external energy into the body. Standing
in the shadows beyond the light of present day science, is the placebo
effect which is capable of producing the desired medical effect
in 60%
of clinical cases overall". In line with my own conviction
as a consumer, Dr Becker has suggested that "ethical
practitioners of minimal-energy techniques not deceive their patients
(but) tell them from the start that they are going to cure themselves
by means of control over their own bodies / destinies"
(14).
Such an approach would empower and ethically
serve both patient and practitioner, yet most homoeopaths apparently
feel intimidated. Dr Weil relates a personal favourable encounter
with homoeopathic treatment and concludes: "I feel comfortable
with the conclusion that the homeopathic remedy functioned as a
placebo". (7) A key concept at a recent conference was that
complementary therapies construct the consultation to give non-specific
factors prominence, where especially symptom relevance and congruence
between health beliefs of the practitioner and the client may be
particularly significant. (15)
Although placebo may be defined
as a treatment that does not have a specific effect on the illness
for which it is being used, or as an intervention for which there
is no scientific theory explaining its mechanism of action, placebo
can be an effective therapeutic intervention. Placebo can
be administered as a drug or as a procedural intervention. Multiple
factors affect the ultimate intensity of the placebo response. One
of these factors is the approach taken by the health care provider
in administering an intervention. The medical literature
is replete with clinical studies showing beneficial results of placebo
administration. Physicians should attempt to better understand placebo
to harness its beneficial effects, avoid nocebo or negative effects,
and maximize the placebo response. (16)
Physicians throughout medical history
knew three possible ways to explain the association between treatment
and cure: 1. the beneficial effect of the treatment itself, 2. the
healing power of nature, and 3. the placebo effect. In
the modern definition by Grunbaum, a treatment is a placebo when
the effect cannot be explained by the theory that describes its
activity. In clinical practice the placebo phenomenon is commonly
misunderstood. Most clinical pain can be reduced to at least half
of its intensity by placebos. Also cough, headaches, asthma and
other ailments can thus be relieved. (17) Explanatory theories
are often much narrower in focus than the phenomenon they seek to
explain
There can be no final verdict on
the efficacy of any, (including all orthodox) treatment
until researchers start to take the placebo effect seriously.
This means evaluating instead of controlling it. Patients might
not mind being given dummy pills engineered to produce a convincing
but harmless array of side effects. (18) The mere act of treatment,
independent of its content, can elicit cures by means of the placebo
response (7). Deliberate use of the placebo response will
maximise patient satisfaction and treatment efficacy. If the placebo
effect could be patented and bottled, it would be worth a fortune.
The placebo effect is an unpopular topic.
In complementary medicine the 'aura of quackery', linked
to any discussion of the placebo effect is for many, too close for
comfort. At a recent conference titled "Placebo: Probing
the Self-Healing Brain" Lawrence Sullivan, a historian of religion
at Harvard Divinity School noted: "Nobody wants to own it.
Even shamans and witch doctors would be offended by the idea that
their healing powers depended on the placebo effect". Harvard
Medical School anthropologist Arthur Kleinman asked: "Why
is the placebo regarded as pejorative? Is it threatening to medicine?"
(19) The author of this and associated reports has no gripe with
homoeopathic practitioners using the homoeopathic placebo to good
effect for self-limiting conditions and minor conditions under their
supervision. It is however considered criminal to treat serious
conditions thus, and to sell otc’s to this end.
References:
(1) Davidson, J. "Mind, Medicine and
the Placebo Effect". Positive Health. 1998, Feb
(2) Davidson, J. Br. Homoeop. 1995; 85,
(3) Gaus, W. Unpublished report. Dept. Biometry,
Univ Ulm, 1994;
(4) Ernst, E."Placebo Effects".
Ch.7 in Homoeopathy: A Critical Appraisal. Edited by Edzard Ernst
and Eckhart Hahn.
Lond. Butterworth-Heinemann, 1998;
(5) "The Power of Magic". Editorial,
New Scientist, 1997, 27 Sept;
(6) Brown, W."The Placebo Effect".
Scientific American, 1997, Jan;
(7) Weil, A. Health and Healing. Warner, 1995;
(8) Strauss, J. and Cavanaugh, S. Psychosomatics,
1996;
(9) Annual N. E. Psychological Assoc. Meeting-
Paper Session-III, 1996;
(10) Verdugo, R and Ochoa, J. J. Neurol Neurosurg
Psychiatry, 1998, Aug; 65(2);
(11) Kirsch, I and Saperstein, G. Prevention
and Treatment, 1998, Article 00002a, 26 June;
(12) Beutler, L. Prevention and Treatment,
1998, Article 00003c, 26 June;
(13) Kirsch, I. Prevention and Treatment,
1998, Article 0007r, 26 June;
(14) Becker, R. Cross Currents: The Promise
of Electromedicine. Tarcher/Putnam, 1990;
(15) "The Placedo Response: Biology and
Belief". Univ. Westminster, 1996, Nov;
(16) Bernstein CN, Placebos in medicine. Seminar,
Gastrointest Disease, 1999 Jan; 10(1):3-7 ;
(17) Bugel, P.The Many Meanings of Placebo.
Forsch Komplementarmed 1998; 5 Suppl S1: 23-30;
(18) "Patient Heal Thyself". Editorial,
New Scientist, 1998, 11 July,
(19) "Placebo: Probing the Self-Healing
Brain". Harvard Univ, 1995, Dec.
|
“A common fallacy” within homoeopathic advocacy
is that “homoeopathy is both safe and effective”.
Director of the Office of Complementary Medicine, US National Institutes
of Health, Dr Wayne Jonas, author of a popular treatise on homoeopathy
(1), reluctantly increasingly a sceptic in the light of developing
research, in an article titled “Safety in homoeopathy”
explains that “The conventional reaction is that they
are all placebo, can have no specific effects at all; that is, either
therapeutic or toxic, and therefore are at least harmless. This
attitude is reflected in the approach taken by the US Food and Drug
Administration, that generally classifies homoeopathic preparations
as over-the-counter drugs approved for sale without claims of effectiveness,
and exempt from the standard toxicity and safety testing required
of other medications”. (2)
Jonas: “If recent evidence indicating
that homoeopathic medications may not work in identical fashion
to placebo, are substantiated, and they produce specific effects,
then the possibility exists that they also may produce specific
adverse effects and their evaluation will require the same assessment
of risk benefit ratio as any other intervention”.
(2) My thesis is that homoeopathic
treatment bears definite risk that a patient with a serious non
self-limiting condition will actually be receiving no effective
extraneous treatment, and is also at iatrogenic risk. Jonas,
corroborates: “treatment with ineffective therapy,
will result in unnecessary progression of disease and adverse effects.
Some homoeopaths claim that there is a duration of action from certain
potencies, even up to a year after a single dose.
The author has seen cases in which individuals with chronic illness,
such as gingivitis and gall bladder disease, have been told to wait
for the full duration of action of the remedy, resulting in continued
suffering”. (2) Similar records exist involving
children, eg treated for atopic dermatitis, pneumonia, cervical
strep-lymphadenitis, and acute lymphatic leukaemia. (3)
Avogadro's law states that above a dilution
of 12C/24D(X), there is unlikely to be a single molecule of the
original substance. As a general rule, low potencies could, according
to the “pharmaco-logical” or “immuno-logical”
potential of the starting substance, produce a measurable effect,
but with the exception of toxic agents, allergens and disease organisms
or innoculants (nosodes/isopathy), higher potencies are unlikely
to exert other than allergenic, let alone claimed beneficial effects.
Loscher concurs: “Homoeopathic drugs may exert pharmacodynamic,
including toxic effects at low dilutions of D0-D6.
There is no scientific effect of higher dilution except
for substances with high toxic potential”.
(4) Low potencies and especially the complexes with indications,
respectively violate 1, 2 and 3 of Hahnemann’s Three Laws
of Homoeopathy.
Definitive study of the adverse effects
of homoeopathic remedies have not been conducted but even if they
are merely placebos, adverse reactions (known as "nocebo effects")
can clearly still ensue from their use. (5) Professor Edzard Ernst,
Chair of Complementay Medicine at Exeter University (UK), believes
that “The assumption that homoeopathy, even though
ineffective, is free of risks, is questionable, since side-effects
and complications associated with homoeopathy have been reported
in the literature, and on the basis of which data the notion of
totally risk-free homoeopathy is untenable”.
(6) Loscher and Richter, Institute of Pharmacology, Toxicology and
Pharmacy in Hannover, Germany, conducting a critical evaluation
of the most important homoeopathic drugs concluded:“Several
of the marketed homoeopathic drugs for treatment of animals represent
a risk for both the animals and the consumer of food produced from
animals”. (7)
Aulas conducted an extensive literature
search, reported and recommended: “Little progress has
been made in documenting the side-effects of homeopathic preparations.
Serious adverse effects have been reported with low dilutions
<4C/8D(X) given parenterally or orally. Homeopathic
preparations should not be used to treat serious diseases when other
drugs are known to be both effective and safe. Regardless of the
condition treated, homoeopathic dilution below 5C/10D(X)
and especially low decimal dilutions must not only be considered
as having no proven efficacy but also as having potential dangers”.
(8)
Products misbranded as homoeopathics may
also work only because of adulteration with therapeutic levels of
eg steroidal drugs. (9) Because it is not mandatory, yet is actionable,
homoeopathic side-effects are rarely sought and/or reported. Homoeopathy
employs numerous extremely toxic substances supposedly in infinitesmal
amounts. However, commercial remedies have been found to
contain toxic doses. By way of one example: “In order to test
the widely held assumption that homeopathic medicines contain negligible
quantities of their major ingredients, six such medicines labelled
in Latin as containing arsenic were purchased over the counter and
by mail order and their arsenic contents measured. Values determined
were similar to those expected from label information in only two
of six and were markedly at variance in the remaining four. Arsenic
was present in notable quantities in two preparations.
No warnings appeared on the labels”. (10) “Acute
pancreatitis following administration of a complex homoeopathic
remedy” has been reliably reported. (11)
Montoya-Cabrera reported: “an
infant with diaper dermatitis and mild respiratory and enteral infections,
treated with a homeopathic mercurial medicine: Mercurius 6a (cinnabar
dilute 1 x 10000000), thereafter became seriously ill with exacerbation
and dissemination of the dermatitis as well as irritability and
albuminuria. Mercury urine levels were 60 micrograms/L (reference
less than 10 micrograms/L)”. An antidote chelating agent was
administered. The clinical conditions improved and urinary levels
of mercury decreased to normal values. The researchers concluded
that “homeopathic medicaments should be recognised as potentially
harmful substances”. (12) Stevens reported: “a case
of human thallotoxicosis, confirmed by faeces analysis, caused by
the taking of a homoeopathic preparation”. The patient
rapidly developed symptoms of thallium poisoning. Antidote treatment
with Prussian blue resulted in recovery. (13)
Prescrire International reported that Austrian
authors (14) recorded adverse reactions in three patients. “The
first, recovering from a 'flu like' syndrome, took a homeopathy
preparation containing compounds in 4 D(X). After three days he
developed pruritis with palmar and plantar oedema followed by erythroderma.
The second developed a measles-like skin rash after taking a complex
botanical homeopathic mixture. The third developed anaphylactic
shock requiring intensive care after taking homeopathic
preparations of pollens. Re-challenge with the associated remedy
was positive in all cases, and show that homeopathic preparations
can induce immuno-allergic reactions without having to be injected”.
(8) Others report similarly, confirming that homoeopathy can produce
dangerous side-effects as seen with orthodox drugs. (15)(16) Also
Apis (crushed bee)(source Hahnemann Homoeopathy Clinic), has resulted
in worsening episodes of back-pain, spreading to other parts of
the complainant’s body; and both Hepar sulph (source unstated)
and Silicea, (source Dolisos), has resulted in anorexia, paresthesia,
psychological and systemic symptoms. (17)
Homoeopathic philosophy raises interesting
questions, eg "Tinctures possess a number of undesired
side effects. Why would only the beneficial effects be amplified
("potentiated"), while all other side-effects would be
attenuated?" (18) This logically leads us to the possibility
that all high potency effects might be adverse effects. Ivons has
warned: “Homoeopaths eagerly anticipate homoeopathic aggravations
which are not always benign. Severe, even life threatening
physical or emotional symptomology is possible in the guise of aggravation.
We do a disservice to the public to tout homoeopathy as absolutely
safe". (19) Dantas and Fisher, in a recent review
of UK proving trials expressed surprise at finding that “most
provings were done because of known properties of medicinal plants“
and concluded:“on the negative side, some recent
homoeopathic pathogenetic trials are unreliable and may be positively
damaging to patients”. (20)
Dr Fredric Motz, Chairman of the Homoeopathic
Association of SA, in a 17 September 1997 submission to Parliament,
clearly stated: “the public is unable to practice homoeopathy,
and this goes for health shops and other health professionals. It
is dangerous to practice homoeopathy without requisite knowledge
and much harm can be done in this way. Arnica can cause fatal haemorrhage
in certain individuals that take blood thinning agents (like Warfarin).
Silica can open up old TB glands with deleterious effects. Phosphorus
given to a bronchial carcinoma can easily lead to death. Caulophyllum
may produce abortion at any stage of pregnancy etc. Much harm comes
also from unqualified people treating or giving advice to sick people
because due to lack of knowledge and diagnostic skill, this could
lead to very dangerous consequences. It is wrong to assume a public
right to self-medicate or buy via OTC, medicine used in homoeopathic
practice”. So even the homoeopaths themselves, or at
the more honest individuals amongst them, agree with my thesis.
Jonas: ”Assessment of safety in
homoeopathy is even worse. Even minimal approaches are usually not
found. When done objectively, it has not indicated an innocuous
nature, even with high dilutions. The author has seen a sudden severe
aggravation of asthma necessitating hospitalisation. Homoeopathic
literature teaches suppression or symptom shifting in which superficial
treatment or symptom control results in deeper and more serious
symptoms arising. Classical literature describes serious suppression
arising from treatment in the hands of incompetent practitioners.
Homoeopaths often see the return of old symptoms as a good sign
rather than an adverse effect. Important issues arise about the
interpretation of return of old pathological conditions, eg whether
old pathologies might also return in serious conditions eg cancer,
asthma or other diseases”. (2) Benmeir et al report how
“a patient with a melanoma, subsequent to exclusive postoperative
treatment with homoeopathic remedies, developed a recurrent tumour
weighing 1.8 kg.” (21)
German researchers report: “Severe
adverse reactions observed in association with homoeopathic remedies,
including need for treatment in an intensive care unit”. Hentschel
et al recently analysed emergency room /intensive care unit admissions
to the Medical Dept at the University of Erlangen to detect causal
relationships between homoeopathic treatment and emergency hospitalisation.
Homoeopathic treatment had been applied for an average of 18.6 days
prior to admission. (In a 1-year period) 63 patients themselves
attributed their complaints to the homoeopathic treatment they had
received. With one exception, all were ‘above’ X 23.”
The shocking conclusion: “The rate of adverse reactions,
39.7 %, is (relatively) high”. (22)
The public naively associate homoeopathy
with wholesome herbs, but in addition to the above-mentioned serious
safety considerations, common remedies often include highly objectionable,
toxic and even disease-sourced causative organisms including cockroach,
bedbug, snake, spider and insect and animal venoms, dog’s
milk, rabid dog’s saliva, cancerous tissue, diphtheria virus,
syphilitic virus, tubercular abscess pus with bacilli, and hundreds
of other agents, including their inevitable combination with their
vehicular milk-sugar tablets and alcohol drops, creating ethical
problems for unsuspecting Jews, Muslims, Sikhs, Hindus and strict
vegetarians and vegans. These products should accordingly carry
mandatory explicit ingredient and warning labels, and in accordance
with the lack of evidence of efficacy, bear no indications / false
therapeutic claims.
References:
(1) Jonas W, Jacobs J, “Healing
With Homoeopathy: The Natural Way to…Health”, Warner
Books 1996;
(2) Jonas W, Ch 9, “Safety in Homoeopathy”,
in Ernst and Hahn (Eds), "Homoeopathy…", Heinemann
1998;
(3) Tsur M, “Inadvertent child health
neglect by preference of homoeopathy”, Harefuah 1992 Feb;122(3);
(4) Loscher W, “Homeopathy: risk-free
alternative?”, DTW Dtsch Tierarztl Wochenschr 1992 Feb;99(2);
(5) Ernst E, “Direct Risks…”,
in Ernst (Ed), Complementary Medicine: An Objective Appraisal. Butterworth.1996;
(6) Ernst E, “Risk-free homeopathy?”,
Schweiz Med Wochenschr 1996 Oct;126(40);
(7) Loscher W, Richter A, “Homoeopathy
in vet.. med..”, Berl Munch Tierarztl Wochenschr 1993 Apr;106(4);
(8) Aulas J, Prescrire International, Homoeopathy
Update, 1995;15(155) (Engl Trnsl 1996 Feb;5(21));
(9) Morice A, “Adulterated homeopathic
cure for asthma”, Lancet 1986 Apr 12;1(8374);
(10) Kerr H, Saryan L, “Arsenic content
of homeopathic medicines”, J Toxicol Clin Toxicol, 1986; 24(5);
(11) Kerr H, Yarborough G, “Pancreatitis
following a homeopathic preparation”, N E J Med 1986; 314(25);
(12) Montoya-Cabrera M, et al, “Mercury
poisoning by a homeopathic drug” Gac Med Mex 1991; 127(3);
(13) Stevens W, “Thallium intoxication
caused by a homoeopathic preparation” Toxicol Eur Res 1978;
1(5);
(14) Aberer W, Strohal R, “Homeopathic
Preparations Severe Adverse Effects”, Dermatologica 1991;
182(4);
(15) Van Ulsen J, et al, “Chromate dermatitis
from a homeopathic drug”, Contact Dermatitis 1988 Jan; 18(1);
(16) Forsman S, “Homeopathy can be dangerous…”,
Lakartidningen, 1991 May;188(18);
(17) US FDA, CFSAN Special Nutritionals /
Adverse Effect Monitoring System Web Report, Oct 20, 1998;
(18) Hopff W, “Is homeopathy a false
doctrine?”, Monatsschr Kinderheilkd 1993;141(3);
(19) Ivons M, Letter, “Who is qualified?”,
Resonance: J International Foundation of Homeopathy 1995;17(5);
(20) Dantas F, Fisher P, Ch 5, in Ernst and
Hahn (Eds) “Homoeopathy: A Critical Appraisal", Heinemann
1998;
(21) Benmeir P, et al, “Homeopathic
Life-threatening Negligence”, Ann Plast Surg 1991; 27(6).
(22) Hentschel C, et al, Ch 10, “Reports
of complications in homoeopathic treatment”, in Ernst and
Hahn (1998).
|