|
|
 |
|
 |

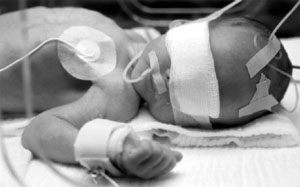
| It seems to be an increasingly common practice these days for the ignorant to take the health of consumers into their hands. Nowhere is this more apparent than in the sphere of so-called natural health and healing, a sector that I have been actively involved with for the past 25 years. Even with personal care products, the area of my professional focus for the past 15 years, there is evident a very real risk of consumers falling prey to harm from such products, not only from the mass-production Cosmetics and Toiletry industries, but alarmingly, also from the so-called natural products sector. The special danger here arises not only from the commercial expropriation of what was once traditionally a highly ethical specialty sector far more focussed on service than profit, but even more sinister, from an element that uses fraudulent ingredient scare marketing propaganda, along with pretence or false belief in superiority over competitors that these operators tend to malign. A case in point is that of Naturebabes / Tom-eTots' use of Cocamidopropyl betaine in preference to SLES and the use of Eucalyptus oil, which is considerably more dangerous than the Parabens. Eucalyptus oil can cause skin irritation and contact dermatitis (Mitchell J, Rook A, Botanical Dermatology, Greengrass Vancouver, 1979); (Spoerke D et al, Vet Hum Toxicol, 31:166, 1989); (Webb N et al, J Paediatr Child Health, 29:368, 1993). Eucalyptus oil is official in the Indian Pharmacopoeia as a counter-irritant (Indian Pharmacopoeia, Vol. 1, Government of India Ministry of Health and Family Welfare, Delhi, 1996) and is official in the Chinese Pharmacopoeia as a skin irritant used in nerval pain (Tu G, ed., Pharmacopoeia of the People's Republic of China, Guangdong Science and Technology Press, Beijing, 1992). Eucalyptus oil furthermore has skin tumour promoting activity (Roe F et al, Food Cosmet Toxicol, 3:331, 1965). Several plant-derived essential oils have epileptogenic properties (without a history of or existing epilepsy), including Eucalyptus oil, which due to its highly reactive monoterpene ketones, is a powerful convulsant. Topical application of Eucalyptus essential oil is recorded in the paediatric medical literature as inducing systemic toxicity, expressing as slurred speech, generalised tonic (muscular contraction) status, ataxia and muscle weakness, and toxic seizure, progressing to unconsciousness with severe complications and poor outcome. Seizures are more frequently reported in children than adults and transient coma can be induced in infants by exceptionally low concentrations. (Chun L, Hawaii Med J, 11(2), 1951); (Darben T et al, Australas J Dermatol, 39:265, 1998); (Burkhard P et al, J Neurol, 246(8), 1999); (IPCS INCHEM, Eucalyptus Oil (PIM031) International Programme on Chemical Safety, - WHO, UN EP, IPCS, 2005) The Medline Plus Medical Encyclopedia lists eucalyptus oil as a poisonous ingredient, the application of which can cause symptoms of muscle weakness, shallow (possibly rapid) breathing, rapid heartbeat, dizziness, drowsiness, convulsions, seizures and unconsciousness, and advise that as emergency home treatment the body be washed with soap and water before calling emergency services. If there is survival past 48 hours, this is usually considered as a good sign that recovery will occur (Johnson C, The Medline Plus Medical Encyclopedia, US Natl Library of Medicine and Natl Inst Health, May 2005).
Eucalyptus oil preparations should be kept out of reach of children and not be applied to the face, especially the nose of infants or young children (Whitman B et al, J Paediatr Child Health, 30(2), 1994); (Blumenthal M et al, eds, The complete German Commission E Monographs, Am Botanical Council, Austin, TX, 1998). So high was paediatric Eucalyptus oil poisoning in the indigenous region of Australasia, that preventive countermeasures were considered, including discouraging vaporiser use for respiratory infections among young children and altogether discontinuing the use of eucalyptus oil as a therapeutic agent (Day L et al, Aust N Z J Public Health, 21(3), 1997). Pediatric preparations incorporating Eucalyptus oil, were officially suspended by the World Health Organisation more than a decade ago (WHO Pharmaceuticals Newsletter, 10:2, 1994) and are now subject to Schedule-5 substance classification in Australia (National Drugs and Poisons Schedule Committee, Meeting 36, Therapeutic Goods Administration, 2002). |
||||||||||||

|
||||||||||||
|
||||||||||||
|
||
| |
||
|
||
| |
||
|
Gaia is copyright © 2006 Gaia all rights
reserved Designed by Webs The Way |
||
|
Page Counter as of January 2008 |