|
|
 |
|
 |
 |
 |
 |
PART
1 |
| PART
2 |
PART
3 |
The following
sentence, is for me (ST), the kicker observation in the abovementioned
article: “In a study of rub-on progesterone applied
to breast tissue, both progesterone "and oestrogen"
levels increased by four times, and yet any blood levels of progesterone
were short-lived (Mauvais-Jarvis
P, Wepierre J & Vickers C, Percutaneous
Absorption of Steroids, Academic Press, NY, 1980)”,
a fact apparently known since the
pioneering days of topical progesterone research (Mauvais-Jarvis
P et al, In vivo studies on progesterone metabolism by human skin,
J Clin Endocrinol Metab, 29:1580-1585, 1969),
but that has been either unnoticed or deliberately ignored by
pro-progesterone cream advocates and merchants. I
have long suspected and now contend that it is not the
so-called ‘natural/bio-identical’ progesterone that exclusively,
or even largely exerts a paradoxical positive effect on menopausal
and female complaints, but a logical direct result of the body
being forced to attempt to return to hormonal homoeostasis by
uprating its synthesis of oestrogen, the specific natural antagonist
to the progesterone sneaked-in via the skin by and so by-passing the body’s
sentinel, the liver, which would ordinarily detoxify the exogenous
progesterone that it would rightly perceive to be an undesirable
xenobiotic. Additional exogenous (external) progesterone
detected in the bloodstream is determined to be counter to the
body’s innate wisdom, which directed and ensured that growth-stimulating
(read carcinogenic) hormonal production be appropriately lowered
in the face of declining need for reproductive fertility against
the ever-increasing risk of malignancy that accompanies life’s
many insults and advancing age. A logical
extension of my hypothesis is that women are delaying the body’s
protection against hormone-induced carcinogenesis - the menopause
(marked by the first non drug-induced missed period). This is
a short sighted trade-off for a short period of extended relative
youthfulness and reproductivity against a tragic life-long thereafter
increased risk of breast cancer, not only from increased exogenous
progesterone-induced endogenous oestrogen, but also exogenously
introduced progesterone, their endogenous carcinogenic metabolites,
their disruption of dynamic natural oestrogen and progesterone
receptor balance and their combined synergistic carcinogenicity.
I have never felt a need to have to set out proof of these mechanisms
– they are after all simple logic. I will however, summarise
extensive evidence of several of the mechanisms involved. |
|
Layout of the evidence |
| I
shall hereafter introduce several concerns raised by Lynne McTaggart
for laypersons in recent issues of her ongoing series ‘What Doctors
Don’t Tell You’ (WDDTY) and also amplify these concerns as articulated
for laypersons and alternative and complementary practitioners
in WDDTY and in specialist peer-reviewed journals, by Dr Ellen
Grant, a physician and
medical gynaecologist currently in private practice, who was a
pioneer critic of the abuse of hormones in medicine and made outstanding
contributions to alerting and educating the general public (The Bitter Pill, Corgi, 1985); (Sexual Chemistry,
Cedar, 1994); (WDDTY 2004, 2005 & 2006) and fellow medical scientists and doctors
via more than 60 published papers and communications since 1962
regarding the risks involved in introducing exogenous natural
and/or synthetic hormones and congeners, in particular oestrogen
and progesterone, into the human body. Grant originally focussed
on oestrogen, later oestrogen and/or progesterone analogues and
recently, the progestagens, including so-called ‘natural’ progesterone.
I will introduce some debate with Dr Lee and his followers to
illustrate examples of their naivity. Finally, I summarise my
additional supporting research showing beyond all reasonable doubt,
that topical/transdermal natural/bio-identical progesterone is
a huge breast cancer risk. In all I have
read of Dr Lee’s writings, only one single ‘original’ statement
qualifies as truth, confirming how ridiculous his hypothesis really
is, since even he admitted that all that is required is a healthy
plant food diet: “Just as with phytoestrogens,
many plants make progesterone like substances. In cultures whose
diets are rich in fresh vegetables of all sorts, progesterone
deficiency does not exist. Not only do the women of these cultures
have healthy ovaries with follicles producing sufficient progesterone,
but, at menopause, their diets provide sufficient progestogenic
substances to keep their libido high, their bones strong, and
their passage through menopause uneventful and symptom free.”
(Lee J,
‘Estrogen Overkill’, WDDTY vol 5 no 4, 1994) |
Researchers at the
Institute of Plant Physiology, Polish Academy of Sciences, Krakow,
Poland, reported:
“Mammalian sex hormones such as 17beta-estradiol (oestrogen) and progesterone were present in 60-80% of the plant species investigated. Enzymes responsible for their biosynthesis and conversion were also found in plants.” (Janeczko A, Skoczowski A, Folia Histochem Cytobiol, 43(2), 2005) Cholesterol in association with low-density lipoprotein is the primary blood-borne precursor used for the body’s synthesis of progesterone, making raw materials for progesterone production an unlikely problem in most reasonably healthy persons. Once progesterone is synthesised by or introduced into the body, it can, via physiologic conversion in the corpus luteum, ovaries, testes and adrenal glands, be synthesised to all of the hormones, including oestrogens, androgens, and corticosteroids. Adrenocorticotropic hormone, synthesised by the anterior pituitary gland, stimulates the conversion of cholesterol into pregnenolone, the immediate precursor of all steroid hormones, including progesterone. (Biochemistry, L Stryer (Ed), WH Freeman & Co, NY, 1995) In humans, the final step in progesterone biosynthesis is the conversion of pregnenolone to progesterone. Pregnenolone is the precursor of progesterone and DHEA and its inhibition disrupts normal steroidogenesis of these essential steroids and in turn, those of which these are precursors. The corpus luteum produces a number of normalcy regulatory progesterone synthesis inhibitors. Sutherlandia herb, heavily marketed as a supplement (another
informed concern of mine), is a conversion inhibitor of both progesterone
and pregnenolone (Prevoo
D et al, Endocr Res, 30(4), 2004). An excess of iron,
carotene and free radicals inhibit progesterone synthesis, as
does a sunlight deficiency. Transdermal progesterone is not a
natural alternative to lifestyle excesses and/or deficiencies.
Industrial chemists can convert a constituent of Mexican yam diosgenin
into progesterone, but only via unnatural chemical pathways. Aberrant
progesterone may, through bio-modulation, affect endogenous progesterone
production. Despite all of these avoidable lifestyle factors,
it is in fact extremely
rare to suffer from an oestrogen excess/progesterone deficiency,
as claimed by advocates of ‘natural’ progesterone
cream. Soon after its purification in
1933, true natural progesterone became widely used in the treatment
of luteal-phase dysfunction, which is appropriate for true luteal
defects causing decreased progesterone biosynthesis. However,
if the defect involved hypothalamic or pituitary dysfunction or
disorders of follicular maturation, then initial treatment was
more appropriately directed elsewhere,
(Ovarian Endocrinology, S Hillier (Ed), Blackwell Sci Publ,
Oxford, 1991), a far more responsible approach than
that of the progesterone cream cowboys. |
| Witness examples of the realisation of this truth and of the hazards of indiscriminate use of even low doses of progesterone cream within of the cautious parameters originally recommended by Dr Lee, but irresponsibly long lost by his followers due to rampant commercial opportunism: |
 |
|
Researchers at the Clinical Pharmacology Research Center at Bassett Healthcare, Cooperstown, New York, following a 2-year study of postmenopausal women using an over-the-counter natural progesterone cream at a network of three hospitals and 21 health centers in central New York, reported: “Many progesterone studies measure serum, which is blood that has had the cells in the blood filtered out. This gives inaccurate results because progesterone circulates in the blood bound to cell membranes. When progesterone reaches receptors in the organs of influence, the breast, uterus or placenta, it detaches from cell membranes and attaches to the receptors. Therefore, in order to be accurate, whole blood must be measured. Our data show that one brand of natural progesterone cream (Pro-gest), using a dosage consistent with the higher dose directions on the cream's label, gave equal exposure to the (5-times higher) therapeutic dose of micronised oral progesterone (Prometrium). The use of topical progesterone without medical supervision is concerning because of the possibility of increased risk of coronary artery disease, stroke, thrombosis and breast cancer.” (Dr Anne Hermann, Presentation: “The Bio-equivalence of Over-The-Counter Progesterone Cream” at the annual meeting of the American Society for Clinical Pharmacology and Therapeutics in Miami Beach, Florida, USA, 25 March 2004) |
This reality is not the exclusive domain of medical researchers. A few responsible practitioners are taking note, doing their own analysis and making significant changes in the use of these products in their practices. Dr Mercola On ‘Complications Regarding Progesterone Cream’ Dr Joseph Mercola,
DO, a peer-reviewed published author and popular online (mercola.com)
natural health advocate takes an informed common sense approach
to topics, including personal experience and reported: “I have also learned that it is far more important to normalize the adrenal hormones first, following which, progesterone levels will frequently normalize in 3-6 months and not require any hormone supplements to keep the balance. The balancing process involves dietary lifestyle changes and regular adequate sleep to stabilise biorhythms. Addressing emotional stress in one's life is the other huge component. Once the lifestyle issues are addressed, one would ideally evaluate the adrenal and female hormones with multiple samples and proper evaluation of the test results and then, if necessary, further balancing the adrenals to recalibrate endocrine control and help the body to start making the hormones by itself, rather than be stuck on hormone treatment for the rest of one’s life.” “I have been finding that many of the women who were on progesterone cream have terribly elevated levels of this hormone. This is repairable, but may involve going off the cream for as long as two years to wash the progesterone out of the system. I am still in an evaluation stage and learning about how common this is in my own practice. I am convinced that this is a big part of the picture for hormone replacement. There will be a huge shift in the way that we are dispensing progesterone cream in our office without evaluation of one's adrenal and female hormones, rather than blindly slapping on progesterone cream without any appreciation of the potential complication or hormone disruptions.” (Dr Joseph Mercola, ‘Complications Regarding Progesterone Cream’, mercola.com, 2004) |
|
Premenstrual Syndrome (PMS) lacks a consensus definition, but 10 psychologic and 10 somatic chief symptoms tend to be listed in the medical literature in order of frequency of occurrence. Complicating the study of the syndrome is that the fact that placebo responses in controlled clinical trials have been as high as 80%, with no long-term satisfactory treatment emerging with various forms of progesterone, the most commonly prescribed, yet controversial therapy for PMS, given that progesterone deficiency has never been proved to be a cause of PMS, nor progesterone to be superior to placebo, some specialists believing that 80% of patients can be treated with stress reduction and dietary modification alone. (Smith S, Schiff I, The premenstrual syndrome: diagnosis and management, Fertil Steril, 52(4), 1989); (Clinical Pharmacy and Therapeutics, E Herfindal, D Gourley & L Hart, (Eds), Williams & Wilkins, 1992) Menopause researchers at the Oregon Evidence-based Practice Center and Oregon Health and Science at the University of Portland reviewed 130 studies of alternative therapies for menopause, including transdermal progesterone cream and described its safety and efficacy for hot flushes and preventives for cardiovascular disease, osteoporosis and breast cancer as “bleak” (Speroff L, Intl J Fertil Womens Med, 50(3), 2005). In a subsequent study of 70 previously completed studies of alternative therapies used to treat menopause-related symptoms, their findings showed that a strong placebo effect was about the only consistent result. (Nedrow A et al, Arch Intern Med, 166(14), 2006) The placebo effect can be especially significant for both conditions. Dr Grant has long ago pointed out that: “Deficiency
of progesterone is unlikely to be responsible for the premenstrual
syndrome as the week following menstruation is usually the time
which is most often free from symptoms and at this part of the
cycle there are very low levels of progesterone (Grant E et al,
Munch Med Wochenschr, 117(38), 1975); (Grant E et al, Minerva
Med, 67(31), 1976).” Should the reader believe themselves
familiar with McTaggart’s and Grant’s WDDTY and BMJ evidence and
are not yet entirely convinced, please proceed to my additional
irrefutable evidence, which proves, via peer-reviewed
published scientific literature, that progesterone is carcinogenic and causes breast cancer. |
|
|||||||||
| In WDDTY,
vol 5, no 5, 1994, Dr Grant responded to Dr John
Lee: “The Second Opinion "Estrogen
Overkill" by Californian general practitioner Dr John
Lee (WDDTY vol 5 no 4) is a
disturbing amalgam of fact and fiction. Progesterone is not
an essential nutrient, which must obtained from our food all
our lives. In contrast, ingested hormones are destroyed in
the gut. Animal studies by Dr Gina Schoental have shown that
even small amounts of extra progesterone, estrogen or testosterone
at key times during pregnancy can interfere profoundly with physical
and mental development and sexual orientation. As a safeguard,
we make our own sex hormones as and when needed during reproduction.
It is impossible to mimic this delicate system by crudely adding
hormones from the outside. For the 30 years ‘natural’ progesterone
has been prescribed. I have personally seen these patients then
suffer from irregular bleeding, severe migraine, depression, weight
gain, leg cramps and painful breasts. All the many well
documented health risks of the contraceptive pill, are due to
it acting predominantly like progesterone (pro-gestation)
which is what ‘progestogen’ or ‘progestin’ means. The estrogen
influence in contraceptive pills is relatively small.” “Much is being made of a new syndrome
(luteal deficiency syndrome or LDS), when women have low post
ovulation levels of progesterone, longer cycles and more risk
of foetal abnormalities and recurrent miscarriages if they conceive.
However, flagging secretions of hormones cannot be boosted
by exogenous oestrogen or progesterone. Adding progesterone, even
as cream, will increase the risk of damage to the mother and baby.
The underlying causes of the condition are often deficiencies
of essential nutrients. Exogenous hormones will only make the
problem worse by causing further zinc and magnesium deficiencies
and eventually deplete copper stores. When zinc is deficient a
woman's own hormone production is impaired. In fact, all
exogenous steroid hormones can block the secretion of the body's
pituitary and ovarian hormones. A varied, low
allergy diet is a safer and more effective way of maintaining
or restoring a woman's hormone production”. “In general, estrogens stimulate immunity, increasing antibody production, while progesterone and testosterone cause immunosuppression at many points on the immune pathways (Immunol Rev, 1984; J of Immunol 1988; 1491:1-8). In fact, progesterone is a more powerful immunosupressant than the adrenal steroids. Progesterone also can act as a co-carcinogen with viruses and chemicals (Potential Carcinogenic Hazards from Drugs, 1967; 7: 162-71, Springer Verlag, Berlin, NY). Both female hormones combine to develop and dilate blood vessels spreading infection and cancer (Sexual Chemistry, 1994, Lancet 1994; 343: 926). Progesterone increases melanin formation, another reason for eschewing skin rubbing (to avoid blotching). Although Lee advocated the use of progesterone to treat osteoporosis, progesterone in DMPA form has been shown to cause the condition (BMJ 1993; 303:13-6). Researchers have demonstrated that osteoporosis is due to nutritional deficiencies.” |
|||||||||
| In
a subsequent edition (WDDTY, vol
5, no 6, 1994), Dr Lee responded to Dr Grant and
she in turn to him: [JL]: “Dr
Ellen Grant (WDDTY, vol 5, no 5, 1994)
claims that natural progesterone supplementation is dangerous.
Her own references do not support this. Indeed, Dr Gina Schoental,
whose animal studies are claimed to show that progesterone "can
interfere profoundly" with development, says she has never
even worked on progesterone! [EG]: “Dr
John Lee fails to understand my work and that of others. Both
progesterone and progestogens produce identical changes in endometrial
cells, enzymes and blood vessels. Both relate to widespread systemic
effects such as headaches and mood changes. Any differences are
of degree and individual sensitivity, not of kind. Dr Schoental studied estrogens, but cites other references that progesterone can cause cancer and
birth defects (Dangerous
Properties of Industrial and Consumer Chemicals, 1994: 581)
and
can change into oestrogen.
For instance, progesterone skin gel induces a fourfold increase in both progesterone
and the oestrogen estradiol (Mauvais-Jarvis,
Percutaneous Absorption of Steroids, 1980).” [JL]: “Regarding
immunosuppression, another study cited by Dr Grant (J
Immunol, 1988; 141: 91) does not actually refer to progesterone,
but only to estrogen and testosterone, whereas Immunol
Rev, 1983; 75: 117 suggests that progesterone prevents
fetal rejection during pregnancy while preserving ‘normal’ systemic
immunocompetence against tumours.” [EG]:
“About immune system suppression, the study quoted actually states that
immune system function is only ‘relatively normal’ in pregnancy.
Progesterone suppresses local immunity by blocking the helper
T-cells and enhancing the production of suppressor cells, so preventing
rejection of the ‘foreign’ fetus.” [JL]: “Emerging
research shows the increasing frequency of premature follicle
depletion in women (probably due to petrochemicals).” [EG]: “The
fact that a 10 fold reduction in migraines can immediately follow
withdrawal of prescribed hormones clearly demonstrates that these
are much more toxic than background petrochemicals (The
Lancet, 1979; i: 581). Removing such causes may restore
ovarian function, but progesterone cream cannot.” [EG]: “Temporary or permanent ovarian failure is caused by early age exposure of some 97 per cent of women to prescribed hormones, smoking and/or severe nutritional deficiencies. Each separately increase cancer risks. A temporary improvement in osteoporosis among some women is not proof of long-term safety. Progesterone induces breast proliferation; prescribed hormones have been increasing cancers for decades while the FDA has approved their use. Dr Ellen Grant, London” |
| WDDTY,
Vol 12, No 2, 2001 reported: “New evidence points
to a strong link between raised progesterone levels and the ‘eventual’
development of breast cancer. Using data from several countries,
researchers analysed progesterone levels in women aged 25-35 during
the mid luteal phase of their cycles (from five to nine days preceding
the next period) and found that during this phase, an increase in
progesterone of more than 70 per cent coincided with a more than
eightfold rise in the rate of breast cancer.” (BMJ,
2001; 322: 586-7). |
| In published correspondence titled: “Medical treatment for menorrhagia and the cult
of progesterone” (Grant
E, Rapid Response [to Jane Burgermeister, Medical treatment for menorrhagia
may only delay hysterectomy BMJ 2004; 328: 730-d], 29 March 2004), Dr Grant strongly cautioned medical practitioners:
“Exogenous hormones have always been the wrong treatment for menorrhagia
in my opinion. Large doses of progesterone may shrivel the endometrium
but can cause a disproportionate development of endometrial blood
vessels and heavy, irregular bleeding (Grant
E, J Obst Gynae Br Com, 74:908-18, 1967); (Grant E, Br Med J,
3:402-5, 1968). My extensive research into the hormone
balance of oral contraceptives and the endometrial vascular changes
in untreated and treated cycles, showed that irregular
bleeding relates to lack of oestrogen or progesterone dominance.
Alternative practitioners have been deluded into believing that
‘natural’ progesterone is safe, while it is only synthetic progesterones
which cause breast and cervical cancer, vascular and mental diseases
and immunosuppression in general. This mistaken belief has developed
a cult status. However, enough progesterone may be absorbed locally to be carcinogenic.
A possible risk for a breast cancer patient, who has already had
a mastectomy, is of contralateral breast cancer if she rubs progesterone
on her remaining breast. Further hormone exposures are known
to increase the likelihood of a second cancer developing. Localised
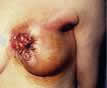
distended veins on the rubbed skin can also happen.” |
| In
published correspondence titled: “Epidemiologist’s
long-term underestimation of harm from hormones”
(Grant E, Rapid Response [to Editorial,
Jan P Vandenbroucke, Benefits and harms of drug treatments, BMJ 2004; 329: 2-3], 3 July 2004), Dr Grant opinioned: “I think it extraordinary
that epidemiologists, who have been consistently miscalculating
the risks of hormone use since the 1960s, to the detriment of
millions of women world-wide, should now be congratulating themselves,
on belatedly getting it right. The original Family
Planning Association’s oral contraceptive trial in the UK, which
was started in 1962, attempted to record every single event. The
results of testing seven progesterones and two oestrogens were
disastrous. It was clear that the formulations most likely to
cause headaches and migraine were also more likely to cause more
serious vascular events like strokes and heart attacks. The endometrial
pathology clearly showed an adverse effect on blood vessels in
women having vascular adverse reactions. In my anonymous
editorial for the BMJ in 1969 (Grant
E, Editorial BMJ 1969; 4; 789-91), I said that it was unlikely
that merely changing strengths and doses of hormones could solve
the problems, because these were an inevitable part of hormone
use with peak complaints varying with different hormone balances.”
“Vessey,
Doll and Sutton published a paper claiming that oral contraceptives
prevented ‘benign’ breast disease
(Cancer 1971: 28: 1395-99). The
‘evidence’ was that significantly fewer longer users had breast
disease. This ignored the fact that sore breasts and vascular
reactions were reasons for early discontinuation. More of the
longer pill takers had breast cancer but the study was then too
small for this to be statistically significant. The flawed ‘prevention’
reasoning prevailed and gave rise to equally spurious claims of
hormone use preventing heart disease and ovarian and endometrial
cancers. Basic research into how hormone use caused immunosuppression,
and therefore an increase in all illnesses, was sidelined and
ignored. An exact “safe” hormone balance was sought for individual
conditions that could be neutralised, even although a progesterone dominant combination is more likely to cause
breast cancer. As oestrogens and progesterones
can have opposite effects on fat levels, years were spent in finding
(seeking) ‘beneficial’ combinations without actually investigating
what was really happening by using the most informative tests.”
|
| |
 |
| In published correspondence titled: “Katharina
Dalton and progesterone dangers” (Grant E, Rapid Response [to S Holton, Obituary: Katharina Dorothea
Dalton, BMJ, 2004;329:1048], 1 November 2004), Dr Ellen Grant wrote: “‘Women are indebted to Dr Greene and Dr
Dalton for so clearly describing their problems in the premenstrual
syndrome’. So began my BMJ review of Dalton's book, ‘The Premenstrual
Syndrome and Progesterone Therapy’ (William
Heinemann Books, Lond, 1977) in 1978 (Grant E, BMJ 1978; 1: 165).
Dalton’s personal charm, energy, enthusiasm and dedication to
her patients, were beyond question to all of us who knew and were
inspired by her. Unfortunately, I failed to persuade
her (Dalton) against the use of progesterone as a ‘therapy’
for premenstrual symptoms. Dalton’s belief that only synthetic progesterones caused side-effects
has achieved “cult” status with the sale of progesterone cream
and promotion of progesterone “therapy” by the late Dr John Lee.”
“Progesterone induces
secretory changes in endometrial glands which increases endometrial
and platelet monoamine oxidase activities (MAO) dramatically,
increases vascular development and causes irregular bleeding and
headaches, and also induces proliferation in breast tissues.
Removal of the ovaries has long been used to prevent endogenous
progesterone production as a treatment for breast cancer. Recent
studies confirm that progesterones cause more breast cancer than
oestrogens. Progesterone is also potentially teratogenic (cause
birth defects). Progesterone-induced high MAO activities
match depressive mood changes in untreated or in treated cycles,
whether symptoms last for a few days premenstrually or continuously
as adverse effects of progesterone use (Grant
E, Pryce Davies J, BMJ 1968; 3: 777-80). Steroid sex hormones may suppress symptoms in
some women by a stress -modulating effect but their mental adverse
effects are often dismissed as “psychological” by hormone prescribing
enthusiasts.” “Ivan Oransky’s obituary in the
Lancet (2004; 364:1576) points
out that Daltons' (negative) vitamin B6 study was questioned in
the Lancet and by other researchers but was influential in the
1997 UK Committee on Toxicity restriction of the sale of vitamin
B6 to 10 mg per day. Dalton’s advice to eat small frequent starch
meals could furthermore cause fluid retention, weight gain and
migraine, especially when combined with progesterone ‘therapy’.
Severe functional deficiencies of B vitamins are common, especially
because (exogenous hormones) cause vitamin B 6 deficiency and
50 mg doses of vitamin B6, along with other B vitamins, are needed
for repletion. Common marked deficiencies in women with PMS are
essential enzymes co-factors like zinc, copper, magnesium and
B vitamins, potassium and essential fatty acids. These basic deficiencies
impair hormone production, receptor activities and alter amine
pathways. Such impairments of homeostatic mechanisms unmask symptoms
when hormone levels fall, even if these levels are already abnormally
high, as HRT tachyphylaxsis demonstrates. (Grant
E, J Nutr Environ Med, 1998; 8: 105-116).” |
| Dr
Grant, in correspondence titled: “Reduction
in mortality from breast cancer: Fall in use of hormones could
have reduced breast cancer mortality” stated
the terrifying truth about exogenous progesterone thus: “In
the Million Women Study and the US Women's Health Initiative
(WHI) study, hormone replacement with progesterone
caused four times more breast cancer than with oestrogen
only. Ten years of progesterone or oestrogen trebles
the risk of breast cancer compared with five year's
use. Changes in hormone use have played a large
part in changes in the incidence of breast cancer mortality
and should not be ignored in studies of the effect
of treatments.” (Grant
E, Lett, BMJ, 330(7498), 2005)
In response to a naturopath (Gilmore
D, Rapid Response to Grant, BMJ, 2 May 2005), who took
exception to the foregoing in defence of ‘natural’ progesterone
cream, claiming it to be “strongly anti-cancer”, Dr Grant
countered as follows: “There is no evidence progesterone
is strongly anti-carcinogenic. On the contrary, progesterone is immunosuppressive
and prevents foetal rejection in pregnancy. Endogenous
progesterone stimulates growth in breast glands during the secretory
phase of an untreated cycle and in pregnancy, just as exogenous
progestogens do. Progesterone and progestagens increase mitosis
and proliferation in breast tissue
(Anderson T et al, Hum Pathol 1989;
12; 1137-43). For nearly 200 years endogenous progesterone
production has been stopped in premenopausal women by bilateral
oophorectomies for the treatment of breast cancer. The first patient I saw who had rubbed progesterone cream on
her breast, developed breast cancer in that breast. Progestogen, progestagen or progestin means acting like
progesterone, different names because they
often have extra actions.”
(Grant E, Rapid Response to Gilmore, BMJ, 3 May 2005) “Naturopaths,
alternative medicine practitioners and nutritionists perhaps believe
that progesterone cream is a kind of universal panacea, as do
many others, a cult status, which seems to be entirely unjustifiable.
Researchers were unable to confirm that applying a quarter teaspoon
of cream containing 20 mg progesterone to the skin daily had any
protective effect on bone mineral density but did confirm a reduction
in vasomotor symptoms (Leonetti H et
al, Obstet Gynecol, 1999; 94: 225-8). This is alarming
evidence that enough progesterone is being absorbed to have
the usual steroid-immunosuppressive effect on menopausal symptoms
in some women. Menopausal symptoms warn of nutritional deficiencies
and allergic reactions and any exogenous hormone use should be
contra-indicated. Chasing the holy grail of suppressing warning
symptoms with HRT has cost the lives numerous women. The Million
Women Study found increases in breast cancer and mortality
with hormone use, irrespective of which type of progesterone was
used.” “Any type of progesterone
can induce endometrial gland atrophy. The fact that progesterone
and progestins induce the same changes in endometrial glandular
enzymes and blood vessels was ignored by Dalton and Lee, as were
my efforts to enlighten them, that acting-like-progesterone means
precisely that as far as important cell enzymes are concerned.
In pregnancy and in a normal cycle the immunosuppressive effects of progesterone
are balanced by high levels of oestrogens. Recent studies are investigating why taking progesterone
caused increases in breast cancer in large prematurely terminated
international trials.
Progesterone- dependent up- regulation of tissue factor, the
initiator of the extrinsic coagulation pathway, is associated
with an enhanced risk of metastasis (Kato S et al, Progesterone increases tissue
factor gene expression, procoagulant activity, and invasion in
the breast cancer cell line ZR-75-1, J Clin Endocrinol Metab,
2005;90:1181-8). It is the same old hormone story -
each disciple believes in special magic formulae for treating
a physiological condition and chooses to disregard the evidence
of much greater harms from using exogenous hormones.” Dr Grant, having despatched with Gilmore’s arguments and references, concluded: “I don’t know why Lee and Dalton’s disciples never see women with adverse reactions to exogenous ‘natural’ progesterone. I have seen many, ever since I was Clinical Assistant to the late endocrinologist Dr Gerald Swyer at University College Hospital. Many women gained weight, developed migraine, depression or irregular bleeding when using ‘natural’ progesterone. Alternative medicine practitioners seem happy to ignore the basic scientific facts that synthetic progesterones act predominantly like progesterone, even if they may also have extra oestrogenic or androgenic actions. Why they feel compelled to dice with women’s lives in this way is a complete mystery to me. Nutritional Medicine is a powerful tool, which makes hormone use unnecessary in my experience. There is nothing natural about exogenous hormones.” (Grant E, Progesterone and synthetics acting like progesterone, Rapid Responses, BMJ, 26 May 2005) |
| To finish my current coverage
from McTaggart and Grant, I jump to the near present, in particular
the cover story of the May edition of What Doctors Don’t Tell You,
titled “Natural Progesterone: The Cancer Risks Revealed”
(WDDTY, vol 17, no 2, 2006).
In her editorial:
Viewpoint: “The end of the debate!” Lynne McTaggart
wrote: “Dr
John Lee believed he had discovered the source of menopausal symptoms,
osteoporosis – indeed, virtually every female problem on the planet.
The culprit, he believed, was ‘oestrogen dominance’ and the solution,
‘natural progesterone’ (administered as a cream). His was a highly
plausible line: today’s women are overwhelmed by environmental
oestrogens, and so the need for progesterone to hormonally balance
themselves. Lee also maintained that since the type of natural
progesterone that he advocated was biochemically identical to
what the body produced, it was safe to use, whereas hormones that
were synthetically produced, were nothing like the real thing
and hence were dangerous. As a result of Lee’s proselytising,
alternative practitioners began to prescribe natural progesterone
with enthusiasm.” “I remained unconvinced.
What bothered me about the story at the time was my discovery
that ‘natural’ progesterone was not a natural anything. It was
a substance made in the laboratory by taking the sterol base of
wild yam and chemically tweaking it, adding molecules here and
there until you produced something with the same molecular blue
print as ovary-derived progesterone.
It was, in other words, a drug. What also bothered me was
that progesterone is primarily a pregnancy hormone.
Women are finished with pregnancy at the menopause.
What is the effect of taking a hormone that you’re not
supposed to need anymore? Finally, I thought about the fact that
progesterone is an immunosuppressant.
High circulating levels of progesterone allow a woman to
carry a foreign protein (ie a foetus) in her body for nine months
without expelling it. Thanks to progesterone, I was no longer allergic to wheat when I
was pregnant. So what
was the effect of taking something over the long term that turns
your immune system down so low?” “Lee over the years, recommended
rub-on cream for more arcane uses: to prevent premature birth,
and as a treatment for reflux and, most dangerously; he began
recommending it to prevent breast cancer. Although most of the
(alternative) medical community embraced Lee’s theory, one lone
wolf besides me remained sceptical. As a young doctor, Ellen Grant was one of the main
UK researches in the first birth control pills of the 1960s and
witnessed first-hand what they were able to do to women.
She denounced the Pill, and for than 40 years went on
to research the effects of exogenous hormones. She was one of
the few people willing to question many of Lee’s basic assumptions.
The fruits of her research
in this month’s cover story offer stark new evidence that Lee’s
simple, well-intended message was not only wrong, but also dangerous. Progesterone of any variety is carcinogenic. Indeed, it is progesterone,
rather than oestrogen, that is the most carcinogenic hormone of
the two. There are two morals
to this story. First,
for all of us in alternative medicine, it’s important to resist
a suspension of all disbelief when it comes to products touted
as natural. Second, a simple and sobering truth: taking
extra sexual hormones at any point in your life is likely to give
you cancer. Hormones are finely turned substances. We tamper with them at our peril.” Over to Dr Ellen Grant, physician,
medical gynaecologist and nutritionist; honorary secretary
- Doctors Against Abuse From Sex Hormones; founder member – British
Society for Ecological Medicine (originally the British Society
for Allergy, Environmental and Nutritional Medicine); and editorial
board member, Journal of Nutritional and Environmental Medicine,
official journal of the Australian College of Nutritional and
Environmental Medicine and the American Academy of Environmental
Medicine, who wrote as follows: “Dr Lee’s main argument was that as the progesterone
cream was ‘chemically identical’ to that produced in the body,
it was safe. The
reality is that even a woman’s own natural endogenous progesterone
is potentially dangerous because it is primitive steroid that
is highly immunosuppressive and potentially carcinogenic.
Progesterone levels are highest during pregnancy and although
it is rare to develop breast cancer at that time, when it does,
it can spread with the speed of an abscess.
In healthy woman, progesterone levels are never high without
high oestrogen levels – either in the luteal phase of the menstrual
cycle or during pregnancy. This
protects against progesterone-induced immunosuppression, which
maintain pregnancy by preventing the rejection of the foreign
paternal proteins in the foetus. Although oestrogens increase
antibody production, progesterone decreases antibody production,
which may be one reason why
progesterone is more carcinogenic for the breast than oestrogen.” “New research using breast cancer cells has discovered that progesterone
encourages breast cancers to spread rapidly and metastasise. Progesterone also induces rapid growth of
leaky blood vessels. Professor Gary Owen in Chile and Professor
Jan Brosens at Hammersmith Hospital, London, discovered that these
breast cancer cells displayed an 18-fold increase in messenger
ribonucleic acid (mRNA) tissue factor (TF) expression after
only six hours of progesterone treatment.
High TF expression is associated with an increased invasive
and metastatic potential of many type of malignancy (J
Clin Endocrinol Metab 2005; 90; 1181-8). TF increases
strongly relate to increased secretion of angiogenic mediators,
such as vascular endothelial growth factor (VEGF), which are also
important in the growth of cancer. TF bound to clotting
factor VII provides protection against cell death, which aids
the development and survival of cancer cells.
Over-expression of TF increases the clotting tendencies
seen in cancer patients.” “The authors
of the study discovered that both
progestin and (or) progesterone cause an increase of epidermal
growth factor (EGF) signalling, which in turn provides a survival
advantage to new cancer cells. It is this increase
in EGF signalling that may contribute to the breast-cancer risk
associated with either endogenous progesterone or with progestin-containing
HRT. According to the authors, their results show that natural progesterone and synthetic progestogens
both have a similar action in the body. This
evidence kicks away the main platform on which Lee built his case
that natural progesterone is somehow safer than synthetic progesterone.
This is hardly surprising as progestogens specifically
imitate the effect of progesterone in the body. The evidence is
now clear - natural progesterone is just another form of HRT.”
“Dr
Sebastian Mirkin and colleagues from Eastern Virginia
Medical School measured the effect of various concentrations
of estradiol, progesterone and synthetic progestogens on two forms
of VEGF using two breast-cancer cell lines, one
composed of cells rich with oestrogen receptors and the other
rich with progesterone receptors.
A positive effect on VEGF would indicate a substance
that promotes cancer. Both progesterone and progestogens increased vascular endothelial
growth factor (VEGF), with the highest doses having the greatest
effect. However,
the natural oestrogen 17-beta-estradiol had no effect in any dose
(Fertil Steril, 2005; 84;485-91).
So the effect of
regular rub-on progesterone (or HRT or the Pill)
is continuous exposure to progesterone (or progestogens)
that stimulates angiogenesis
over longer periods than occurs in the secretory phase of a normal
menstrual cycle. Increased angiogenesis leads to cancer.” “Professor
Maurizio Cutolo, Genoa, Italy, has studied the way
in which sex hormones modify the immune system. Progesterone
inhibits immune cell growth and increases cell death, while oestrogens
protect against cell death and increase antibody formation. He
is concerned that, in both men and women with autoimmune diseases,
sex hormones in peripheral tissues convert into very high levels
of carcinogenic oestrogen metabolites (Rheum
Dis Clin North Am, 2005; 31: 19-27). Giving progesterone
makes matters worse. The first patient I saw who had used progesterone
cream was an American, who after she had a mastectomy for breast
cancer, rubbed progesterone on her remaining breast but soon developed
a second breast cancer. The final irony is that progesterone
can cause many of the symptoms menopausal women are trying to
alleviate - vaginal dryness (painful sexual intercourse),
vaginal thrush, and cervical cancer. The
menopause is nature’s way of protecting women from the dangers
of raised levels of progesterone.” |
.jpg) |
|
In response to
Dr Grant’s “Cancer in a Cream?” article, Virginia Hopkins,
a co-author with the late Dr Lee, who died of a heart attack in
October 2003 at age 74, responded in ‘A Special Edition of the
Hopkins Health Watch’ on the Official Website of John R Lee, MD,
I am tempted to respond to the points made, since the commercially
vested interest fora on which this and other hysterical outbursts
have greeted the unwelcome truth are unlikely to host dissenting
views as generously as WDDTY has done. I shall however resist
commenting on what are essentially emotionally immature ravings
and intellectually bankrupt rehashings of long ago scientifically
defeated arguments by the multifarious followers of Dr Lee and
the ideological and lucrative commercial activities they defend
at all cost. I might just add that as a fellow anti-fluoridationist,
I admired Lee’s writings on that subject and even his early writings
on natural progesterone, echoing as they did, the writings of
Raymond Peat PhD. It is lamentable that John Lee is not able to
reign in over-enthusiastic and irresponsible abuse of his natural
progesterone legacy in the face of scientific progress. Virginia Hopkins
wrote: “Dr. Lee greatly admired the early and pioneering work
Dr. Grant did exposing the first birth control pills as dangerous,
and he felt she had been instrumental in galvanizing drug companies
to create safer oral contraceptives, probably saving thousands
of lives in the process. The fact that Dr. Grant is now attacking
someone who isn’t here to defend himself speaks volumes, but there are many of us who are here to defend Dr. Lee and set the record straight.”
Thousands of doctors in clinical practice are turning to bioidentical
hormones because they’re safer and work better.”
How
ridiculous is this? Dr Grant never attacked Dr Lee, just his steadily
faltering message. It is ludicrous in the extreme to expect a
campaign intended to save millions of lives from abuse of unregulated
‘natural’ progesterone to be halted just because Dr Lee is no
longer there to defend himself from an imaginary attack on his
person. As for the fact that thousands of doctors are now once
again engaging in a lucrative promotion just another form of deadly
HRT foolishly substituted for another, but this time under the
misapprehension that progesterone is entirely safe, renders Dr
Grant’s totally non-vested interest efforts highly commendable.
Another argument is progesterone’s reproductive criticality. Yes,
but so are quantity, cycles and stage of life. Hopkins continued:
“After the factual errors (there were none, just wishful thinking
- ST), which cast a shadow over all of Lynne McTaggart and Dr.
Grant’s assertions (only in the minds of Lee’s ’defenders’ – ST),
is the premise that one can declare ‘progesterone causes breast
cancer’ based on in vitro (test tube) research with a
couple of breast cancer cell lines. Breast cancer researcher Dr
David Zava explains, ‘The research Dr. Grant cites is good,
solid scientific work, and very interesting, but it is not even
close to enough information to declare that progesterone is carcinogenic’ Even
Dr Lee’s sometime supporter, Dr Zava, supports the accuracy of
the research cited by Dr Grant, but suggests that it falls short
of proving McTaggart’s and Grant’s declaration that progesterone
is carcinogenic. If Dr Grant’s evidence was indeed insufficient
to support her bold conclusions, allow the proceedings of the
following meeting and my own database futher below to |
 |
| Before
moving to input other than McTaggart’s and Grant’s, which futher
to McTaggart’s preface statement that “Dr Grant has the ‘last’
word about new evidence of cancer risks”, note that the
British Society for Ecological Medicine is hosting a ground-breaking
international meeting at the Royal College of General Practitioners,
titled: “Why Does Progesterone Cause More Breast Cancer Than Oestrogen?”
on 19 November 2006 (we will update with
coverage in due course). Speakers and topics include:
* Dr Sebastian
Mirkin. Eastern Virginia Medical School, Norfolk, USA: Effect
of progesterone/progestins on vascular endothelial growth factor
VEGF mRNA in breast cancer cells. Angiogenesis in breast cancer. * Professor
Jan Brosens, Reproductive Sciences, Imperial College, London:
Progesterone/progestin breast carcinogenesis –tissue factor TF
gene expression, procoagulant activity & invasion in breast
cancer cell line. |
 |
| Note on Chemical Study Materials: Kindly take cognisance of the fact that, contrary to the oft-repeated criticism by progesterone proponents to the effect that epidemiological studies are irrelevant because they do not utilise so-called natural/bio-identical progesterone, the experimental studies to be presented here do use human cells (an in some cases relevant rodent studies) and do use bio-identical progesterone, and still are likely to be stupidly criticised as not utilising human populations, yet these cell studies are ethical, are significantly informative of the risks of exogenous progesterone and suffice as increasing proof of the risks until eventually quantified by epidemiological human studies of bio-identical progesterone, whether by the registrants of such products, the manufacturers or suppliers of unregistered products, regulatory agencies or by independent researchers. The risk data and evidence is already substantial and is increasing exponentially. For rather obvious reasons, past studies considered the adverse effects of various progestogens in oral contraceptives, fertility drugs or hormone replacement therapy that the study populations were using and more importantly, adverse effects that no manufacturer or supplier of unregistered so-called bio-identical progesterone products are prepared to undertake – yet vigorously deny any likelihood without even looking and it is not like the writing has not been on the wall for the past decade and including the present moment. Progesterone proponents are now clearly on the skids and any previously valid criticisms no longer apply. The only valid criticism is that which proponents are exclusively guilty of - need for their own clinical trials, without which, they have no right to claim any safety or efficacy, let alone peddle their toxic wares as safe. |
|
||
.jpg) |
| Whether my earlier hypothesis proves correct or not, transdermal progesterone remains a risk, since both ‘natural oestrogens and progesterone’, are equally scientifically classified as being “reasonably anticipated to be human carcinogens”. (World Health Organization, International Agency for Research on Cancer, IARC Monographs Programme on the Evaluation of Carcinogenic Risks to Humans, WHO, Vol 72, 1999); (National Toxicology Program, Report on Carcinogens, Eleventh Edition, NTP, 2005) If my previous hypothesis is correct, then we have a doubly tragic genocide situation with progesterone. |
 |
An
authoritative government agency summary of the Carcinogenic
Substance Profile for Progesterone (natural /
bio-identical) (National
Toxicology Program, 11th Report on Carcinogens, 2005)
reported: Direct
Carcinogenicity: “Progesterone is
‘reasonably anticipated to be a human carcinogen, based on
‘sufficient evidence of carcinogenicity in experimental
animals (IARC 1982). When
progesterone was implanted subcutaneously (which is where it migrates from topically - ST), mammary
carcinomas were induced at a significantly earlier age and at
a higher incidence in female mice. Long-term subcutaneous implants
induced ovarian granulosa cell tumors or endometrial stromal sarcomas
in female mice (IARC 1974, 1979).
Subcutaneous injections of progesterone induced increased incidences
of mammary tumors in adult female mice and lesions of the vaginal
or cervical epithelia and genital tract lesions in newborn female
mice. Hyperplastic alveolar-like nodules and other dysplasias
were also induced in female neonatal mice (IARC
1979). Long-term subcutaneous injections in female dogs
induced endometrial hyperplasia, inhibition of ovarian development,
marked mammary hyperplasia, and some fibroadenomatous nodules
of the mammary gland (IARC
1979, 1982)”. Editorial
Note:
Co-carcinogenicity is an important toxicological assessment
tool, since the phenomenon is responsible for novel or increased
carcinogenicity of one substance in combination with another. “Female mice injected subcutaneously with progesterone
showed decreased latent periods for the induction of mammary tumors
by 3-methylcholanthrene.
Ovariectomised female mice receiving injections of progesterone developed
sarcomas of the uterine horn when given an intrauterine implant
of 3-methylcholanthrene and developed increased incidences of
squamous cell carcinomas of the cervix or vagina when treated
intravaginally with 7,12-dimethylbenz[a]anthracene (IARC
1974, 1979). Local applications of 3-methylcholanthrene
and subcutaneous implantations of progesterone induced increased
incidences of vaginal-cervical invasive squamous cell carcinomas
in female mice (IARC 1979).
Rats receiving subcutaneous or intramuscular injections of progesterone
had decreased latent periods and/or increased incidences of mammary
tumors induced by oral administration of 3-methylcholanthrene
or 7,12-dimethylbenz[a]anthracene, but only when the known carcinogens
were administered first. An increased incidence of mammary tumors
was induced in female rats fed 2-acetylaminofluorene in the diet
and injected intramuscularly with progesterone. Newborn female
rats receiving a subcutaneous injection of progesterone and a
subsequent intragastric instillation of 7,12-dimethylbenz[a]anthracene
developed increased incidences of mammary adenocarcinomas (IARC
1979).” “No adequate human studies of the relationship between
exposure to progesterone and human cancer have been reported (IARC
1974, 1979, 1982)” (because no-one until recently embarked
on such studies, which is mandatory for registered steroid hormones,
including natural progesterone, but averted by peddlers- ST).
Exposure “Progesterone is a naturally occurring steroid hormone
produced endogenously by all mammalian species. Human placental extracts, of which progesterone
is the main constituent, have been used in preparations for cosmetic
use (IARC 1979). Potential
consumer exposure through dermal
contact could occur from use of these cosmetics. Animal meat
may contain an average of 0.33 mg progesterone/kg if the animal
was treated with a veterinary progesterone implant and consumers
could potentially be exposed by ingestion. Potential occupational
exposure to progesterone may occur through inhalation and
dermal contact during its production or formulation into pharmaceuticals.”
Conclusion “Progesterone is
‘reasonably
anticipated to be a human carcinogen’. Progesterone
in topically applied hormone-containing drugs for over the counter
use is “no longer considered generally recognized as safe (GRAS)
and effective
(National Toxicology Program, Report
on Carcinogens, 11th Edition, NTP, 2005).” Text References: IARC. 1974. Sex Hormones. IARC Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Humans,
vol. 6. Lyon, France: International Agency for Research on Cancer.
243 pp. IARC. 1979. Sex Hormones (II). IARC Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Humans,
vol. 21. Lyon, France: International Agency for Research on Cancer.
583 pp. |
 |
 |
| The fact that endogenous natural and exogenous bio-identical progesterone is carcinogenic is now beyond scientific doubt. Undetermined are the doses at and circumstances under which the risks are excessive for consumers and who assumes responsibility for their protection. The State of California requires that the Governor annually revises and publishes a list of chemicals known to cause cancer. |
 |
|
The
most recent list under Proposition 65,
includes
both Oestrogen and Progesterone (the natural and synthetic forms)
as chemicals known to be capable of causing cancer and likely
to do so in humans,
so in progressive
California,
topically
applied bioidentical progesterone products must already state:
"WARNING:
This Product Contains A Chemical Known To The State of California
To Cause Cancer."
(Proposition 65 List of Chemicals, State of California EPA,
Office of Environmental Health Hazard Assessment, Chemicals Known
to Cause Cancer or Reproductive Toxicity, September 29, 2006)
Since transdermal natural/bio-identical progesterone proponents are willing
to accept Dr Lee’s and other’s assurances that oestrogens (or
at least synthesised oestrogens and their mimics) are carcinogenic,
I am not going to labour the point that even natural healthy human
body-produced oestrogens can be carcinogenic, since their classification
as such also by the IARC and the NTP, undisputed world authorities,
ought to suffice as proof for all but the most ignorant or criminal
New Age health gurus and their unfortunate gullible victims. What
I shall expand on is the authoritative scientific proof that progesterone
is indeed also carcinogenic, and in particular, is implicated
in the aetiology of breast cancer, and as a result of such proof,
that the transdermal natural/bio-identical progesterone cream
gurus and peddlers claiming absolute safety and recklessly
advocating its indiscriminate and unmonitored use for all and
sundry are indeed criminal fraudsters. |
| Note on Animal
and Human Cell-Line Studies |
.jpg) |
|
Remaining
with pure bio-identical progesterone, let us review the research
evidence chronologically.
|
| Since the early 1990’s, evidence already existed indicating that progesterone was not completely safe. (Pike M, Spicer D, Endogenous estrogen and progesterone as the major determinants of breast cancer risk: prospects for control by "natural" and "technological" means. In: Hormonal Carcinogenesis (Li J, Nandi S, Li S (Eds), Springer-Verlag, NY, 209-216, 1991). |
 |
| Researchers from the University of Southern
California, in an authoritative United States government agency
report unambiguously reported: “Mitogenesis and mutagenesis are major driving forces in neoplastic development. The ovarian hormones, estrogens and progesterone, are major effective (direct or indirect) breast cell mitogens.” (Spicer D, Pike M, J Natl Cancer Inst Monogr, (16):139-47, 1993) |
 |
|
“There is overwhelming evidence that ovarian hormones play a crucial role at all stages in the development of breast cancer. Both major ovarian hormones, estradiol (estrogen) and progesterone, play important roles in increasing breast cancer risk. Key epidemiological evidence is that early menopause reduces risk. Therefore, the hormonal pattern of premenopausal women - cyclic production of relatively large amounts of 17/3-estradiol (estrogen) and progesterone - causes a greater rate of increase in risk of breast cancer than the pattern of postmenopausal women - constant low estradiol and very low progesterone. In postmenopausal women, serum estradiol levels are approximately constant at roughly one third of the lowest premenopausal level, and serum progesterone levels are effectively zero.”
“For many years, the increased risk was thought to be solely due to the elevated levels of estradiol (estrogen) in the premenopausal period, and progesterone was considered anti-estrogenic. This is now known to be incorrect. The rate of increase in the breast cancer incidence curve slows considerably after menopause, but the incidence continues to increase. This strongly suggests that whatever happens to increase incidence is, in most instances, not reversible, ie, that factors which increase risk at any particular time will probably cause lifelong increased incidence rates.”
“There has been much debate in the past 15 years over the precise effects of these two hormones on breast epithelial cells. In addition to effect of age at menopause, other epidemiologic observations provide valuable information on the relation of ovarian hormones to breast cancer risk, and when these are considered together with information from studies of breast cell mitotic activity, the nature of the relation of estradiol and progesterone to breast cancer risk can be largely understood. The relation of weight to breast cancer risk is critically dependent on age. Postmenopausal breast cancer incidence increases about 2.1 percent per year of age. Premenopausal obesity decreases risk and postmenopausal obesity increases risk.”
Cell Proliferation and Carcinogenesis
“Many carcinogens act solely by increasing cell proliferation. Recent advances in the molecular genetics of cancer provide for cell division being essential in the complex process of the genesis of human cancer. An agent that increases mitotic activity also increases the probability of converting DNA damage - both exogenously and endogenously induced - into mutations. Copying errors and more profound DNA changes, including reduction to homozygosity of tumor suppressor genes, also occurs more frequently with increased cell division. The actions of the natural ovarian hormones - estradiol (estrogen) and progesterone - (and hormones used in ERT and OCs) on the breast do not appear to be genotoxic, but they do affect breast cell division rates and their effects on breast cancer rates are explicable in terms of this mechanism.”
“It has long been accepted that hormones can be cancer promoters via increased cell proliferation. Initiation is, however, also affected by cell division rates. Considerations of ever/never use of particular exogenous hormones are of little value. Cells that have undergone some changes on the way to a full neoplastic genotype may respond differently than normal cells to a particular hormonal milieu. Many of the genes that are altered during carcinogenesis are growth factors, growth factor receptors, signal transducers, or transcription factors. In the postmenopausal period, when estrogen levels are low and progesterone is absent, rates of breast cell proliferation are very low, strongly suggesting that estrogen alone induces some breast cell division, but the mitotic rate pattern over the menstrual cycle suggests that estrogen and progesterone together induce much more cell division.”
The ‘Estrogen Augmented
by Progesterone Hypothesis’ and Key Breast Cancer Risk
Factors “An ‘estrogen augmented by progesterone hypothesis’ provides a highly satisfactory explanation for most of the epidemiologic observations on and the key epidemiologic hormonal risk factors for breast cancer. Early menopause reduces the risk of breast cancer by reducing levels of both estrogen and progesterone. Increased anovulation and frequency of low progesterone levels in the luteal phase (progesterone values are, on average, approximately half of "normal" values) that are associated with premenopausal obesity markedly decrease breast exposure to progesterone, while bioavailable estradiol(estrogen) appears to be almost unchanged during ovulatory cycles and decreased during anovulatory cycles.”
Steroid Hormone Mechanism of Action
“The effects
of estrogen and progesterone on promotion of proliferation and
cell differentiation in normal breast epithelium and breast cancer
cells are mediated through transcriptional activation of specific
sets of genes recognized by their particular receptor proteins.
The function of the hormone-binding domain in the absence of hormone
appears to be inhibitory, preventing the receptor from binding
to its response element. A considerably higher proportion
of cells express progesterone receptor than express estrogen receptor.
Receptor expression in the normal postmenopausal breast is higher
for epithelial cells expressing estrogen receptors than expressing
progesterone receptors, which is the opposite of the situation
in the premenopausal breast. Both
major ovarian hormones, estradiol (estrogen) and progesterone,
play important roles in increasing breast cancer risk.”
(Pike M et al, Epidemiol
Rev, 15(1), 1993) |
 |
|
Researchers commissioned by the
Division of Health Promotion and Disease Prevention Institute
of Medicine, Washington DC, reported: |
 |
A researcher
with the Molecular Biology Program at the University of Colorado
Health Sciences Center, Denver, USA, reported: “Conventional wisdom holds that the mechanisms by which estradiol and progesterone regulate the proliferation and differentiation of uterine epithelial cells, apply equally to the breast. This is inaccurate. Considerable evidence suggests that in the epithelium of the breast, progesterone, like estradiol, has a strong proliferative effect and physiological levels of endogenous circulating progesterone may serve to enhance breast cancer growth and that progesterone antagonists may be powerful new tools for the management of metastatic breast cancer because they block the local effects of endogenous progesterone on breast cell proliferation.” (Horwitz K, Background Paper, Antiprogestins and the Treatment of Breast Cancer, Appendix B, In Donaldson M et al, Antiprogestins: Assessing the Science and Recommending a Research Agenda, Institute of Medicine, Washington, DC, National Academies Press, 1993) |
 |
|
Researchers at the Cancer Research Laboratory
and Department of Molecular and Cell Biology, at the University
of California, Berkeley, USA reported: |
  |
|
| Researchers in the Departments
of Medicine and Pathology, Biochemistry, Biophysics and Genetics
and Molecular Biology , at the University of Colorado Health Sciences
Center, Denver, Colorado, USA reported: “Progesterone is neither inherently proliferative nor anti-proliferative. Progesterone is tissue-specific capable of stimulating or inhibiting cell growth, depending on whether treatment is continuous or transient.” (Groshong S et al, Molec Endocrinol, 11(11), 1997) |
|
  |
|
Researchers
with the Cancer Research Program at the Garvan Institute of Medical
Research, St. Vincent’s Hospital, Sydney, New South Wales,
Australia reported: “Many cancers of steroid hormone target tissues, eg the breast, retain steroid responsiveness. Studies using breast cancer cells in vitro and rodent mammary tumors in vivo suggest that the antitumor activity of antiprogestins is mediated by inhibition of proliferation, but other responses, including induction of differentiation and apoptosis are also apparent after several days’ antiprogestin treatment.” (Musgrove E et al, Molecular Endocrinology 11(1), 1997) |
|
  |
|
Researchers
at Westmead Institute for Cancer Research, University of Sydney,
NSW, Australia, reported: “There is evidence in support of a role for progesterone in cell proliferation in the breast. Growth factors and growth factor receptors have been proposed as candidate mediators of progesterone effects on cell proliferation. Progesterone also increases insulin receptor expression in cancer cells. Co-treatment with progesterone and insulin results in a synergistic induction cancer cell growth, suggesting that the progesterone-mediated increase in insulin receptor expression may result in greater sensitivity to the mitogenic effects of insulin. This is consistent with the ability of progesterone to potentiate insulin effects on synchronously growing breast cancer cell cultures. These effects have been postulated to have negative implications for the therapeutic use of progesterone, since growth-stimulatory effects may be seen in breast tumors that express elevated levels of insulin receptors and IGF receptors.” (Dinny Graham J, Clarke C, Endocrine Rev, 18(4), 1997) |
|
 |
| Researchers at Brown University
School of Medicine and Rhode Island Hospital, Providence, USA reported: “The second major hormone affecting the breast is progesterone. Progesterone increases mitotic activity, making cells more susceptible to random genetic errors and to the influences of carcinogens. Progesterone levels are highest during the luteal phase of the menstrual cycle. The argument for progesterone's role in breast cancer development centers on the known risk factors of early menarche and late menopause. With more total ovulatory cycles (thus, more luteal phases) in a lifetime, the odds of a random genetic error are increased.” (Mustafa I, Bland K, Ann Surg, 228(5), 1998) |
  |
|
| Researchers from the Department of Medicine,
University of Colorado Health Sciences Center, Denver, USA reported: |
|

 |
|
Researchers
in the Department of Cell Biology at Baylor College of Medicine
and the Department of Integrative Biology, Pharmacology and Physiology
at the University of Texas–Houston Medical School reported: “We have shown for the first time that ICI 182,780, which is generally considered to be a pure antiestrogen, also blocks transcriptional activation by progesterone and exhibits potent antiprogestin activity. Our results clearly demonstrate that this drug almost completely abolishes the effects of physiological levels of progesterone and hence represents a possible therapeutic mechanism in the treatment of breast cancer.” (Nawaz Z et al, Cancer Research 59, 372-376, 1999) |
|

 |
|
Researchers
in the Department of Cell Biology at Baylor College of Medicine,
Houston, Texas, reported: “To define the functional relevance of progesterone-initiated intracellular signaling in mammary gland tumorigenesis, the progesterone receptor knockout (PRKO) compared with isogenic wild types (WT) mouse model was used in the context of an established carcinogen-induced mammary tumorigenesis system [7,12-dimethylbenz(a)anthracene (DMBA)]. The removal of progesterone receptor function resulted in a significant reduction in susceptibility to DMBA-induced mammary tumorigenesis. Our work confirmed and extended those studies that previously implicated a pivotal role for progesterone in mammary tumor development.” (Lydon J et al, Cancer Research 59, 4276-4284, September 1, 1999) |
|
  |
|
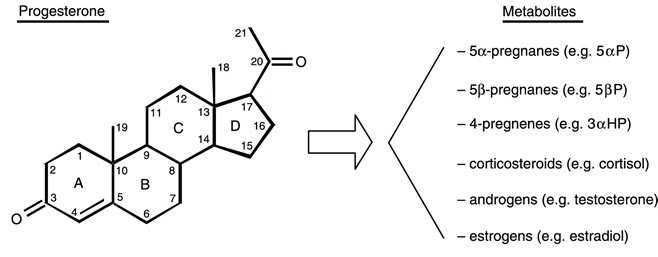
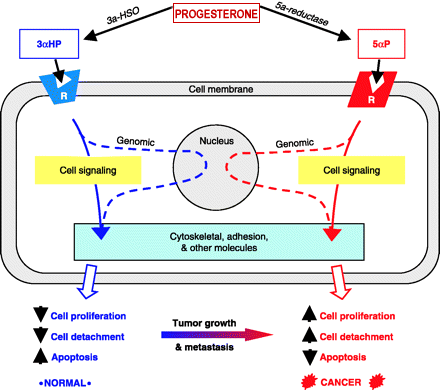
| Researchers at the Hormonal
Regulatory Mechanisms Laboratory, University of Western Ontario,
reported: “The progesterone metabolite, 5alpha-pregnane-3,20-dione (5alphaP), is produced at higher levels in tumorous breast tissue and promotes cell proliferation and detachment. This is the first report of receptors for the progesterone metabolites, 5alphaP and 3alphaHP, of their occurrence in breast cancer cell membranes, and of the induction of 5alphaP receptors by estradiol. The results provide further support for the importance of progesterone metabolites in breast cancer.” (Weiler P, Wiebe J, Biochem Biophys Res Commun, 272(3), 2000) |
|

 |
|
| Researchers at the Hormonal
Regulatory Mechanisms Laboratory, University of Western Ontario,
reported: “To determine whether breast cancer is attributable to progesterone metabolites, we compared the capacity of non-tumorous and tumorous breast tissue to convert progesterone and then tested the effects of these metabolites on breast cell proliferation and anchorage in tissues from the operated breasts of six patients with infiltrating duct carcinomas. The results show that tumorous breast tissue has elevated 5alpha-reductase activity, which results in significantly higher total levels of 5alpha-pregnanes, especially 5alpha-pregnane-3,20-dione (5alphaP). The results suggest that a change in in situ progesterone metabolism, resulting in an increased 5alpha-pregnane:4-pregnene (especially 5alphaP:3alphaHP) ratio, may promote breast cancer by promoting increased cell proliferation and detachment. Progesterone metabolites may provide a new hormonal basis for breast cancer.” (Wiebe J et al, J Cancer Res, 60; 963-943, 2000) |
|
 |
| Researchers at the Center for
Public Health, University of New South Wales, the Department of
Anatomical Pathology, St Vincents Hospital, and the Division of
Virology, Prince of Wales Hospital, Australia, reported: “In vitro studies of human breast cancer in cell lines have shown that administration of oestrogen followed by progesterone stimulates the expression of human endogenous retroviruses in the genome.” (Lawson J et al, Breast Cancer Res, 3:81–85, 2001) |
 |
| Researchers at the Hormonal Regulatory
Mechanisms Laboratory, University of Western Ontario, reported: “Tumorous human breast tissue readily converts progesterone to 5a-pregnane-3,20-dione (5aP), which metabolite has been shown to stimulate proliferation and decrease adhesion of MCF-7 breast cancer cells. Our study suggests that decreases in adhesion and increases in cell proliferation following 5aP treatment may be owing to depolymerization of actin and decreased expression of actin and vinculin. We conclude that the progesterone metabolite 5aP may be involved in promoting breast neoplasia and metastasis by affecting adhesion and cytoskeletal molecules.” (Wiebe J, Muzia D, Endocrine, 16(1), 2001) |
 |
| Researchers
at the Institute of Public Health, Jagiellonian University, Poland
and the Institute of Community Medicine, Norwegian Cancer Society,
Faculty of Medicine, University of Tromso, Norway reported: “We documented a strong, positive relation between mean progesterone concentrations in premenopausal women from five populations and risk of breast cancer. There are data supporting an oestrogen plus progestogens relation with breast cancer risk. Epithelial cells of the breast have the highest mitotic activity in the luteal phase of the menstrual cycle when progesterone production peaks. The use of oestrogen plus progesterone increases risk of breast cancer to a greater extent than does oestrogen alone. The reduction in breast cancer risk observed among obese premenopausal women and among women with polycystic ovary syndrome is most likely a result of frequent anovulatory menstrual cycles and impaired progesterone production. Thus, a causal link between progesterone and breast cancer risk is biologically plausible.” (Jasienska G, Thune I, BMJ, 323:1002, 2001) |

 |
|
Researchers
at the Department of Molecular and Cellular Biology, Baylor College
of Medicine, Houston, Texas, reported: "Epidemiological studies have shown that early onset of menarche, delayed entry into menopause, cycle periodicity, nulliparity, and a late first pregnancy represent individual risk factors for breast cancer. However, early menopause and early first parity decrease this risk. Progesterone's presence or absence directly influences the establishment of each of these reproductive endocrine states, implicating a stochastic multistep progression in the development of this disease, which achieves its highest rate during the reproductive years of premenopause. By providing a temporal window of opportunity for the progressive acquisition of genetic errors. As a result of these errors, the transformed mammary epithelial cell is predicted to undergo unchecked clonal expansion to a mammary neoplasm. Prolonged progesterone exposure, either through uninterrupted cyclical ovarian activity or by postmenopausal supplementation, has been linked to increased breast cancer risk." (Soyal S et al, Breast Cancer Res, 4(5), 2002) |
|
 |
| Researchers at the Institute of Biological
and Experimental Medicine in Buenos Aires, Argentina reported: “Progesterone and estradiol, and their nuclear receptors, play essential roles in the physiology of the reproductive tract and the mammary gland. The classic view (namely, estrogens = proliferation and progestins = differentiation) has been extrapolated to systems other than the endometrium, such as the mammary gland, and has contributed to a long-held belief that estrogens are the main steroid hormones implicated in the induction of breast cancer. However, this concept has been challenged by growing experimental evidence and clinical data, which points towards progesterone and its related signalling pathways as important players in the induction, progression and maintenance of the neoplastic phenotype in the mammary gland. Estrogens have traditionally been considered associated with an increased risk of breast cancer. However, there is now compelling evidence that progesterone plays an important role in breast cell proliferation and cancer.” (Lanari C, Molinolo A, Breast Cancer Res, 4(6), 2002) |
 |
Researchers
at the Hormonal Regulatory Mechanisms Laboratory, University of
Western Ontario, Canada, using natural/bio-identical 14C Progesterone
(acquired from Perkin Elmer Life Sciences, Ontario) specifically
reported as follows: “In
hormone-related cancers (breast, prostate,
endometrium, testis, ovary, thyroid and osteosarcoma), both
endogenous and exogenous hormones |
| provide the stimulus along the cancer progression pathway. (Henderson B, Feigelson H, Hormonal carcinogenesis, Carcinogenesis, 21:427–433, 2000). Human tumorous breast tissue metabolises progesterone. Our studies suggest that during the breast cancer progression pathway, change in progesterone metabolizing enzyme expression, and hence enzyme activity profile, occur in affected breast tissues. Our studies on breast cell lines further suggest a link between tumorigenicity and increased 5a-reductase activity. Specifically, 5a-reduced metabolites (5a-pregnanes, shown to stimulate cell proliferation and detachment) are produced at a significantly higher rate. The resulting increases in tumor and metastasis promoting and concomitant decreases in inhibitory progesterone metabolites may provide the stimulus for progression and malignancy of breast tumors.” (Wiebe J, Lewis M, BMC Cancer, 3:9, 2003) | |
 |
| Researchers
in the Hormonal and Reproductive Epidemiology Branch, Division
of Cancer Epidemiology and Genetics, at the National Cancer Institute,
Rockville, Maryland, USA reported: “Many hormones, in particular estrogen and progesterone, affect breast tissue and breast cancer risk may reflect the integrated effects of these exposures over time.” (Althuis M et al, Cancer Epidemiol Biomarkers Prev, 13; 1558-1568, 2004) |
 |
| Researchers led by Professor P Crosignani
at the University of Milan, Italy, reported: “Experimental animal data and analytic human epidemiological studies indicate that estrogen and/or progesterone promote some types of mammary tumours. Both accelerate the rate of breast epithelial cell division, thereby increasing the risk of critical mutational changes. Increasing cell synthesis also may enhance the survival of genetically damaged cells, leading to promotion of breast cancer. Both hormones may have an influence at any time during the 15–19 years from mutation to clinical diagnosis of breast cancer (Cutuli B et al, Radiother Oncol, 59, 247–255, 2001).” (European Society of Human Reproduction and Embryology Capri Workshop Group, Human Reproduction Update 10(4), 2004) |
 |
| Researchers at the David and
Alice Jurist Institute for Biomedical Research and the University
of Medicine and Dentistry of New Jersey, Hackensack, New Jersey,
USA: “Although hormones produced in the body are normally beneficial, these can in some circumstances act as carcinogens or carcinogen facilitators. In some such cases increased amounts or changes in the formation of its metabolites might be responsible. In other cases the timing of hormone release plays a critical role. In some cases the hormone acts independently, while in others two or more compounds act in concert to promote cancer. Of the various hormones produced in the body, only aldosterone does not cause cancer!” (Bradlow H, Sepkovic D, N.Y. Acad Sci, 1028:216-232, 2004) |
 |
Researchers at
the Hormonal Regulatory Mechanisms Laboratory, University of
Western Ontario, reported: |
(tumorous and non-tumorous) breast tissues from 11 patients. Expression
of 5alphaR1 and 5alphaR2 was higher in the tumor as compared to
normal tissues.
The
mean tumor to normal expression ratios
was 35-85-fold higher in tumor than in normal tissue.
Changes
in progesterone metabolizing enzyme expression levels help to
explain the increases in mitogen/metastasis inducing 5alphaP progesterone
metabolites found in breast tumor tissues.”
(Lewis M et al, BMC
Cancer, 4: 27, 2004) |
|

 |
|
| Researchers
in the Departments of Medicine, Surgery, Obstetrics and Gynaecology,
and Preventive Medicine, at the Keck School of Medicine, University
of Southern California, and the Department of Pathology, Olive
View-UCLA Medical Center, University of California, Los Angeles,
reported: “Progesterone plays an essential role in breast development and in breast cancer formation. The loss of catabolic enzymes in breast cancer leads to increased progesterone levels, which, in combination with increased progesterone receptors, could enhance progesterone-responsive gene expression and thus metabolites of progesterone may have unsuspected effects on cell growth. Transient or sequential administration of progesterone causes cells to enter a mitotic cycle with induction of genes associated with cell growth. The majority of studies in animals and humans find that estrogen alone does not promote cellular proliferation, whereas estrogen plus progesterone does promotes proliferation of cancer.” (Ji Q et al, Cancer Res, 64, 7610-7617, 2004) |
|

 |
|
Researchers
in the Department of Biomedical Sciences, University of Missouri,
Columbia, and Department of Medicine, University of Colorado Health
Sciences Center, Denver, Colorado, USA reported: “Vascular endothelial growth factor (VEGF) is a potent angiogenic growth factor that promotes growth, expansion, and metastasis of breast cancer. Proliferation of many breast cancer cells is under control of the estrogen and progesterone. Two progesterone receptor isoforms, PRA and PRB are expressed in most human breast cancer cells. PRB predominantly regulates expression of VEGF in breast tumor cells and PRB-enriched tumors express a higher level of VEGF and give tumors a significant growth advantage if stimulated by hormone. Both estrogen and natural progesterone and synthetic progestin stimulates PRB-dependent VEGF expression and a consequence is greater tumor expansion.” (Wu J et al, Cancer Res, 64(6), 2004) |
|

 |
Researchers in the
Department of Molecular and Cellular Biology, Baylor College of
Medicine, Houston, Texas, reported: “Progesterone receptors are expressed exclusively in the mammary epithelium. Progesterone receptor-expressing cells are segregated from proliferating cells in normal mammary glands and the proliferative responses of the ductal and alveolar epithelium to progesterone are associated with local induction of PR-dependent growth factors that act in a paracrine manner on PR-negative cells to control their proliferation. Segregation of the steroid receptor expressing cells from proliferating cells is lost in mammary epithelial cells that have been exposed to carcinogen and in cells of breast tumors and this aberrant change in pattern of receptor expression contributes to abnormal growth of breast cancer cells.” (Mulac-Jericevic B, Conneely M, Reproduction. 128: 139-146, 2004) |
 
|
|
| Researchers at
the Department of Biomedical Sciences, University of Missouri, Columbia,
USA reported: “The proliferation of breast cancer cells is controlled by estrogens and progesterone. We evaluated the pathways involved in regulating vascular endothelial growth factor (VEGF) in response to progesterone and synthetic progestins in breast cancer cells. Angiogenesis, the formation of new blood vessels, is essential for tumor growth, expansion, and metastasis. VEGF is one of the most potent angiogenic growth factors and influences permeability, survival and proliferation of endothelial cells. Hypoxia, oncogenes, pH, and steroid hormones are stimuli known to induce VEGF expression in tumor cells. Breast cancer cells metabolise progesterone and synthetic progestins to compounds that may trigger a growth response and increase VEGF production. The synthetic progestins are less easily metabolised than the natural compound. Our findings show that both natural and synthetic progestins induce VEGF expression and secretion and facilitate increased angiogenesis, cellular proliferation, tumor growth and increased risk of breast cancer.” (Wu J et al, Molec Endocrinol, 19(2), 2004) |
|
 |
Researchers
at the Hormonal Regulatory Mechanisms Laboratory, University of
Western Ontario, reported: “The promotion of breast cancer appears to be related to changes in the in situ concentrations of cancer-inhibiting and cancer-promoting progesterone metabolites.” (Wiebe J et al, Steroid Biochem Molec Biol, 93(2-5), 2005) |
 |
Researchers
at the Hormonal Regulatory Mechanisms Laboratory, University of
Western Ontario, reported: “Our results show distribution of 5alphaP-R in several cell types and provides further evidence of the significance of progesterone metabolites and their novel membrane-associated receptors in breast cancer stimulation.” (Palwak K et al, J Steroid Biochem Mol Biol, 97(3), 2005) |
 |
Researchers
at the Institute of Biological and Experimental Medicine in Buenos
Aires, Argentina and the US National Institutes of Health, Bethesda,
Maryland USA reported: “During the past few years the progesterone receptor (PR) pathway has emerged as a likely player in the pathogenesis of breast cancer, with growing experimental as well as clinical evidence pointing to its protagonistic role. Most of the epidemiological evidence for mammary carcinogens reflects prolonged exposure to female hormones, including progesterone. Our findings provide the first evidence that blockade of PRs using antisense oligonucleotides induces inhibition of tumor growth and provide further evidence for a critical involvement of the stimulatory effects of the PR pathway in mammary cancer.” (Lamb C et al, Breast Cancer Res, 7(6), 2005) |
  |
|
Researchers
in the Department of Medicine, Division of Hematology, Oncology,
and Transplantation at the University of Minnesota Cancer Center,
Minneapolis, USA reported: “Ovarian steroid hormones such as estrogen and progesterone are critical for mammary gland development and are implicated in the development and progression of breast cancer. In vitro, both estrogen and progesterone cause proliferation of cell lines derived from human breast tumors, implicating progesterone in disease etiology. In this report, we show that the growth-promoting effects of progestins are due to progesterone receptor’s function as an activator of cytoplasmic kinase cascades rather than its direct activation of transcription. Co-treatment with peptide growth factors, an accurate reflection of the in vivo environment of breast cells, can change cells’ response to progesterone receptor ligands, making progesterone and progestins highly proliferative agent.” (Skildum A et al, Molec Endocrinol, 19(2), 2005) |
|
 |
| Researchers in the Departments of Surgery,
of Obstetrics and Gynaecology and of Physiology, at the Feinberg
School of Medicine, Northwestern University, Chicago, Illinois,
USA, reported: “The normal non-lactating breast is subject to cyclical changes in response to ovarian stimulation with either estrogen alone during anovulatory cycles, or estrogen plus progesterone during ovulatory cycles. These hormones increase breast epithelial cell proliferation, with progesterone having a synergistic/additive effect during ovulatory cycles. The withdrawal of hormones with the onset of a new ovarian cycle causes a wave of apoptosis, eliminating defective cells and maintaining the population balance of the epithelium. Continuous proliferation, however, leads to an accumulation of genetic defects, with the eventual emergence of a transformed cell, leading to the clonal evolution of malignancy. Therefore, factors that increase availability of hormones, augment the proliferative response of the epithelium to hormonal stimulation, or interfere with apoptotic elimination of defective cells, will increase breast cancer risk.” (Khan S et al, Endocrine-Related Cancer, 12(3), 2005) |
 |
| A researcher at Baylor College
of Medicine, Houston, Texas, USA reported:
“Breast cancer is a major cancer of women, with approximately 210 000 new cases annually in the United States and accounting for approximately 32% of new cancers diagnosed in US women. The death of 40 000 women due to invasive breast cancer remains a sobering fact and indicates the need to understand this disease in greater depth. The logic and rationale for understanding the molecular basis for hormone-mediated breast cancer are based on the consistent observations in human epidemiological studies and the strong confirmatory experiments in rodent breast cancer models. Reproductive history is the strongest and most consistent risk factor outside of genetic background and age.” “Early menarche, late menopause, parity, and late age
of first pregnancy are each independent risk factors. Early age
of menarche translates into earlier hormone exposure to estrogen
and progesterone and to breast epithelial cell growth. The ovarian
hormones, estrogen and progesterone, play a pivotal, paradoxical
role in normal and neoplastic development of the mammary gland.
Long duration of estrogen and progesterone
are associated with increased breast cancer risk, while
short duration of pregnancy level doses are associated with reduced
breast cancer risk. These
hormones induce alterations in gene expression in the mammary
epithelial cells, which
persist for a long time after the hormones are withdrawn from
the host.” (Medina
D, Endocrine-Related Cancer, 12 (3), 2005) |
  |
|
| Researchers at Westmead Institute
for Cancer Research, University of Sydney, NSW, Australia, reported: “After
short exposure to progesterone, transcriptional profiles were
largely unaffected by marked alterations in relative expression
of PRA and PRB. However, after
longer exposure to progesterone, a different repertoire of genes
were regulated and PRA predominance conferred progesterone responsiveness
to a distinct subset of transcriptional targets.
Progesterone effects on cell shape and adhesion are dependent
on the PRA/PRB balance. Our data suggest that an imbalance of
PRA and PRB levels results in remodeled progesterone responsiveness
and that altered
regulation of morphology and adhesion are important components
of altered progesterone response, which may have important implications
for breast cancer biology.”
(Dinny Graham J et al, Molec
Endocrinol, 19(11), 2005) |
|
  |
|
| Researchers at the Department
of Biomedical Sciences, University of Missouri, Columbia, USA reported:
“Angiogenesis, the formation of new blood vessels, is essential for tumor expansion. We have previously shown that natural progesterone and synthetic progestins induce the synthesis and secretion of vascular endothelial growth factor (VEGF), one of the most potent angiogenic growth factors known, in a subset of human breast cancer cells. Many human breast cancers are dependent on sex steroids and much emphasis has been placed on the role of estrogens in the proliferation. We have revealed progesterone induction of vascular endothelial growth factor (VEGF) in cells that contain the progesterone receptor (PR) and mutant p53 protein. Since approximately 50% of all breast tumors are PR positive and 50–60% of all breast tumors carry p53 mutations, it is critical to investigate the functional role of progesterone-induced VEGF in promoting angiogenesis and breast cancer cell growth.” “We discovered
that progestin-induced VEGF increased the proliferation not only
of human endothelial cells, but also of breast tumor cells in
an autocrine and a paracrine manner. Breast cancer cells
were treated with either progesterone or the synthetic progestin
MPA, which is used in HRT. Exposure to either, significantly increased
proliferation. The results suggest that the VEGF produced by tumor
cells was sufficient to cause a proliferative response of endothelial
cells either by itself or in combination with certain tumor cell-
or endothelial cell-derived factors for which VEGF was the predominant
partner. These
results indicate that tumor cells can produce angiogenically active
VEGF in response to natural and synthetic progestins,
which can
subsequently increase the proliferation of human endothelial cells.” |
|
  |
|
Researchers
at the Reproduction and Development Unit and the Department of
Obstetrics and Gynaecology, Faculty of Medicine at the Pontifical
Catholic University of Chile; researchers at the Institute of
Reproductive and Developmental Biology, Imperial College and Hammersmith
Hospital, London, UK; and researchers at the Institute
and Department of Medicine and Division of Endocrinology at the
University of Colorado Health Sciences Center, USA, recently published
a peer-reviewed study and concluded that: “Tissue
factor (TF) mRNA and protein are up-regulated by progesterone
in breast cancer cell lines. TF is the initiator of the extrinsic
coagulation pathway and is associated with metastasis in
a wide variety of cancers. Progesterone
increases glucose transporters, which corresponding increase
in glucose uptake could provide the extra energy required
by the breast cancer tumour for potential angiogenesis and
metastasis. Because TF expression is associated with
an enhanced risk of metastasis, we postulate that the progesterone-dependent
up-regulation of TF provides a survival advantage to burgeoning
breast cancer.” “TF mRNA
gene expression following progesterone treatment may
play a significant role in breast cancer, where elevated
TF, the secretion of angiogenic mediators such as vascular
endothelial cell growth factor (VEGF), which confers enhanced
proliferation, as well as TF bound to its ligand FVII,
which provides protection against apoptosis, as well as procoagulant
activity (thrombosis) due to the presence of high levels of circulating
progesterone, all correlate with increased invasion of cells
(incidence of metastasis) and poor cancer prognosis. Although
co-administration of progesterone counteracts the mitogenic action
of exogenous estrogen on the endometrium, it increases
dramatically the incidence of breast cancer.” “In further regard to this hypothesis, of direct clinical relevance is the timing of surgery in premenopausal women with breast cancer. If performed during the luteal phase of the menstrual cycle, any breast cancer cells that escape into circulation during this procedure have an enhanced metastatic and procoagulant activity due to the presence of high levels of circulating progesterone. This would increase the risk of tumour reappearance and induce a hypercoagulable state, both related to poorer patient prognosis. The majority of breast cancer deaths are attributable to metastases and thrombosis. Based on the correlation presented herein among TF expression, invasion (metastasis), and procoagulant activity (thrombosis), this work presents a mechanism for the clinical observation that the presence of progesterone correlates with the increased risk of breast cancer incidence.” (Kato S et al, J Clin Endocrinol Metabol, 90(2), 2005) |
|
 |
Researchers in the Department
of Physiology at Michigan State University, USA, reported: “Progesterone regulates proliferation and differentiation in the normal mammary gland in mouse, rat and human, but has also been implicated in the etiology and pathogenesis of human breast cancer. The focus of this review is on the role of the progesterone receptor and functional significance of PR isoforms in human mammary cancer.” (Aupperlee M et al, Breast Dis, 24:37-57, 2005-2006) |
 |
| Jennifer Eng-Wong, a medical oncologist with the National Cancer Institute’s Center for Cancer Research, believes that: “the data indicates that progesterone may fuel breast cancer growth". (National Cancer Institute, US National Institutes of Health, Posted 03/13/2006) |
 |
Researchers
at the Hormonal Regulatory Mechanisms Laboratory, University of
Western Ontario, reported: “Progesterone metabolites such as 5alpha-dihydroprogesterone promote both mitogenic and metastatic activity in breast cells.” (Weibe J et al, J Steroid Biochem Molec Biol, 100(4-5), 2006) |
 |
| Researchers with the Jones
Institute for Reproductive Medicine at the Eastern Virginia Medical
School, Norfolk, Virginia, USA reported: “We determined the effect of 17beta-estradiol, progesterone and the progestin, medroxyprogesterone acetate (MPA) on vascular endothelial growth factor (VEGF). The oestrogen, 17beta-estradiol had no effect. Progesterone (natural) and MPA (synthetic progestin) both individually increased vascular endothelial growth factor in breast cancer cells and these differential effects may be related to breast cancer growth.” (Mirkin S et al, Intl J Gynecol Cancer, 16 (Suppl 2):560-3, 2006) |
  |
|
Researchers at the Department
of Biomedical Sciences, University of Missouri, Columbia, USA
reported: |
|
 |
||
| Researchers
at the Hormonal Regulatory Mechanisms Laboratory, Department of
Biology, University of Western Ontario, Canada, recently published
a milestone Review of Progesterone
Metabolites in Breast Cancer (Wiebe J, Endocrine-Related Cancer,
13(3), 2006), summarising the latest findings
as follows: “In addition
to the reproductive tissues and adrenals, every other tissue
that has been investigated appears to have one or more progesterone-metabolizing
enzyme. In the past, the actions of the progesterone
metabolizing enzymes generally have been equated to
a means of reducing the progesterone concentration in
the tissue microenvironment and the end products were dismissed
as inactive waste metabolites. We propose rather,
and review evidence, that these progesterone metabolizing
enzymes act directly on progesterone to produce
two types of autocrines/paracrines with opposing regulatory
roles in breast cancer and with the promotion of
breast cancer related to changes in in situ concentrations of
progesterone metabolites.” “In vitro
studies on a number of breast cell lines indicate that
the progesterone metabolite pregnenes, promote normalcy
by down-regulating cell proliferation and detachment, whereas
the metabolite pregnanes, promote mitogenesis and metastasis by
stimulating cell proliferation and detachment.
The hormones influence opposing actions on mitosis,
apoptosis, and cytoskeletal and adhesion plaque molecules
via cell signalling pathways. Depending on prevailing enzymes
activity/expression, the ratio of pregnenes to pregnanes
is high in normal tissue and the ratio is reversed
in breast tumour tissue and tumorigenic cell lines
because of altered progesterone metabolizing enzyme activities/expression.” “Current
estrogen-based theories and therapies based on suppressing estrogen
levels or inhibiting its actions apply to only a fraction of all
breast cancers; the majority (about two-thirds) of
breast cancer cases are estrogen-insensitive
and have lacked endocrine explanations. Only
a fraction of all breast cancer patients respond to
current endocrine estrogen-targeted therapy
and the response is only temporary. Mammary tissue
in particular has enzymes that catalyze conversion
of progesterone to active metabolites. As
the metabolites 5
Progesterone metabolism in breast tissues and breast cell lines “Recent
studies provide evidence that progesterone metabolites formed
in breast tissue have regulatory functions with
respect to breast cancer that may previously have been
attributed to progesterone, whereas progesterone serves as
a precursor (or pro-hormone) and the metabolites as
the active hormones within the tumour and its adjacent
host tissue. In terms of neoplasia, the presence
of progesterone-metabolizing enzymes has been demonstrated
in rat testicular interstitial cell tumors, androblastoma
(Sertoli-Leydig cell tumor), dimethylbenz(a)anthracene-induced
rat mammary tumors, human endometrial carcinoma,
human breast tissues, modified breast cancer cell lines
and virally transformed adrenocortical cells.” Changes
in progesterone metabolite ratios and metabolizing enzyme activities
“Studies
to determine the capacity of tumor and surrounding
normal breast tissues to metabolise progesterone, using
tissue specimens from premenopausal, menopausal and postmenopausal
women with various subtypes and grades of infiltrating
duct carcinomas and including tissues that were estrogen-receptor
and progesterone-receptor negative and/or positive,
confirmed that all biopsies examined converted progesterone, via
5 “Metabolism
studies showed that the altered metabolite ratios was due to
significantly elevated 5 “Enzyme
activity and expression studies show that both 5 Cancer- related actions of
the progesterone metabolites “Uncontrolled
cell proliferation is one of the hallmarks of cancer and factors
that affect cell proliferation rates affect cancer rates. Studies
conducted on MCF-7 cells showed significant effects
on cell proliferation, with 5 “Increases
in cell numbers can result not only from increased rates
of cell division, but also from decreases in rate of cell
attrition via programmed cell death (apoptosis). A
balance of proliferation and apoptosis provides homeostasis
in normal tissues and alteration in this balance sets off
changes ultimately leading to malignancy. Studies
on cell lines using several methods of evaluating apoptosis
and proliferation/mitosis showed treatment with 5 Effects
of progesterone metabolites on cell adhesion “Cellular
adhesion is a critical aspect of cancer biology. Some time during
the development of most types of human cancer, pioneer
cells are spawned that are capable of moving out of
the primary tumor masses to distant sites. It is these distant settlements of tumor
cells — metastases — that are the cause of about 90%
of human cancer deaths. To allow the initial
escape, adhesion must be decreased and attachment severed.
Tests on MCF-7, MCF-10A, T47D and MDA-MB-231 cells
showed that 5 Proof of principle
“Confirmation
of the hypothesis that the move from normalcy to neoplasia
in breast cells is influenced by the in situ increase in
the 5 Effects
of progesterone metabolites on cytoskeletal and adhesion complexes
“The transformations
in morphology, replication, and adhesion during the
transition from normal to cancerous cells have been shown
to be accompanied by rearrangements of cytoskeletal and adhesion
structures. The cytoskeletal organization differs between
normal and cancerous cells and between high- and low-metastatic
cells. Vinculin is a protein associated with cell-to-cell
and cell-to-substrate adhesion sites. In normal
cells, vinculin may be readily detected, while in highly
malignant cell lines its organization may be significantly
altered or it may not be detected at all, suggesting progression
of malignancy. Treatment
of MCF-7 cells with 5 Receptors for progesterone metabolites in human breast cells Localization,
characterization and Regulation of progesterone metabolite receptors “The actions
of hormonal steroids require complexing with specific-binding
sites (receptors) on target cells. Our studies show
that binding of 5 Role
of progesterone metabolites in regulating ER levels
“Estradiol
can influence mitogenicity of estrogen receptor-positive mammary
cells. Therefore, regulation of estrogen receptor levels
may be important for the progression of estrogen-dependent
mammary neoplasias. Estradiol and progesterone are
known to play a role in modulating estrogen receptor concentrations.
To determine if progesterone metabolites affect estrogen
receptor levels, MCF-7 cells were exposed to 5
Effect of the progesterone metabolite, 5 “The
location of the receptors for 5 |
||
Implications of progesterone-metabolizing enzymes for synthetic progestin-based
contraceptives and hormone-replacement therapy drugs “The synthetic
progestins used for contraception and hormone replacement
therapy (HRT) do not behave like progesterone in terms of their
metabolism and probably not with respect to their actions
at the level of the breast tissue microenvironment.
As different formulations may exhibit marked differences
in chemical structure, metabolism, and pharmacodynamic
actions, it is not possible to generalize about them.
To ascertain the possible role of the contraceptive
and HRT drugs in breast cancer regulation via the progesterone
metabolites, it will be necessary to measure their levels
and composition in the breast microenvironment to determine
their effects on progesterone metabolism in breast tissue
and/or cell lines and to establish whether the progesterone-metabolizing
enzymes can further alter the drugs to pro- or anti-cancer
moieties.”
“Mammary gland
cells show cyclicity and respond to steroid hormones. Normal
breast tissue goes through cycles of imbalance between proliferation
and apoptosis during menstrual periodicity, pregnancy, and
lactation, but regularly corrects these temporary imbalances.
In cancer, changes have occurred such that overall increases
in cell numbers continue and result in the development of
tumors. The changes are due to the changes in
concentration of the ovarian hormones, estradiol (estrogen)
and progesterone. Since estrogens have been shown
to increase proliferation in some cells, and because
about one-third of breast cancer patients show some
responses to anti-estrogen therapies, estrogens have been
considered the primary hormonal cause of breast cancer. In
time, however, estrogen-sensitive neoplasms become unresponsive
and the patients experience relapse. Overall, this means
the existence of an overwhelming majority of breast
cancers for which the current estrogens based explanations
and therapies are inadequate.” “Since
progesterone is involved in normal cyclical changes,
it too has been implicated in breast neoplasia. The progesterone
metabolites, produced within breast tissues, are put forward
as candidates that could up- regulate mitogenic and
metastatic processes in mammary tissues, resulting
in progression to cancer. The suggestion is based on
the evidence provided and summarized in the figure
below:”
“The
work on the potential role of progesterone metabolites in promoting
cancer of the breast is in its infancy and a number
of issues need to be addressed. All the observations
summarized in this review about the effects/actions of the
progesterone metabolites were made in vitro on breast
cell lines in culture. To substantiate the hypothesis
that the progesterone metabolites play a role in mammary
cancer, it is necessary to demonstrate their effects
in vivo. Evidence that progesterone metabolites can
have similar effects in vivo has come from a pilot
experiment conducted by Drs R Schillaci and P Elizalde (NRC, Buenos Aires), who
showed
(personal communication) that C4HD cells developed into substantial
palpable tumors if treated with 5 |
||
Further
evidence of the carcinogenicity of progesterone and its metabolites
(without the progesterone there are no metabolites) will be presented
in due course, both retrospective and also as the data is generated.
There is still time for those promoting natural/bio-identical
progesterone to withdraw their endorsements without becoming knowing
accomplices to the massive silent genocide underway.
Sincerely Stuart Thomson |
| TO BE CONTINUED...... |
| (For update alerts please subscribe to our Newletter here) |
| |
||
|
||
| |
||
|
Gaia is copyright © 2006 Gaia all rights
reserved Designed by Webs The Way |
||
|
Page Counter as of January 2008 |